Description
Have you ever considered what will happen if toxic ions like lead move through your body? They can be stored in your bones and disrupt the body’s proper function. The good news is that there is a solution for this problem. Let me tell you about disodium ethylenetetraacetic acid, which is also known as disodium EDTA.
This chemical substance is a powerful chelating agent. It can bind with metal ions such as lead, mercury, and iron to get them out of your body. So this substance is widely used in lead poisoning. It is interesting to note that other industries also use it in the formulation of cosmetics, fertilizers, and food products.
How to buy disodium EDTA?
If you would like to know any further information about disodium EDTA and its price, our team at Shanghai Chemex will be delighted to help you with that.
Physical and Chemical Properties
EDTA-2Na appears as a colorless crystalline powder that is able to freely dissolve in water. The most important physical and chemical properties of this material is summarized in the table below:
| Name | disodium ethylenetetraacetic acid |
| Chemical formula | C10H18N2Na2O10 |
| Molecular weight | 372.24 g/mol |
| CAS number | 6381-92-6 |
| Flash point | >100 °C |
| Melting point | 242 °C |
| Chemical Structure | 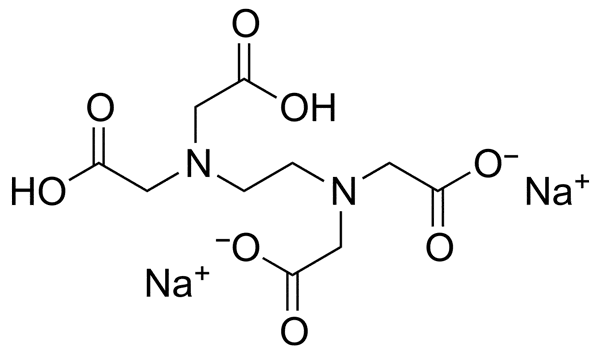 |
Due to its properties, Disodium EDTA can easily prevent the unwanted reactions of metal ions.
How to Prepare Disodium EDTA?
Ferdinand Munz was the first person to produce this material by the reaction of ethylenediamine and chloroacetic acid. Nowadays, EDTA is synthesised from the reactions of ethylenediamine, formaldehyde, and sodium cyanide. This process is used annually to produce 80,000 tonnes of this compound.
H2NCH2CH2NH2 + 4CH2O + 4 NaCN + 4 H2O → (NaO2CCH2)2NCH2CH2N(CH2CO2Na)2 + 4NH3
(NaO2CCH2)2NCH2CH2N(CH2CO2Na)2 + 4 HCl → (HO2CCH2)2NCH2CH2N(CH2CO2H)2 + 4NaCl
Disodium EDTA uses
This salt is used in a wide range of different industries, which are described below:
Food
EDTA-2 sodium is used as an additive in a wide range of products, including canned fish, shrimp, canned mushrooms, and fried potatoes. It is also added to salad dressings to prevent dryness.
Cosmetics
Disodium EDTA can play a role in increasing the effectiveness and impact of products on the skin. It is used in moisturisers, skin care products, cleansers, bath soaps, shampoos and conditioners, hair dyes, lotions, and other products. This chemical can control the viscosity of materials. In fact, the presence of this substance in cosmetics prevents them from spoiling prematurely and completely stops the growth of fungi and other microorganisms.
“Disodium EDTA and the related ingredients bind to metal ions which inactivates them. The binding of metal ions helps prevent the deterioration of cosmetics and personal care products. It also helps to maintain clarity, protect fragrance compounds, and prevent rancidity.”
Textile industries
In the textile industry, EDTA-2 sodium removes free and harmful metal ions, prevents discoloration of fabrics, and also avoids the deposition of materials in industrial equipment operating at very high temperatures. In general, EDTA reduces the reactivity of metals and prevents any side effects that may occur in their presence.
Pharmaceutical industries
It is used as a treatment for lead poisoning. This chemical substance is able to increase blood flow. Some doctors also believe that EDTA acts as an antioxidant and prevents free radicals from attaching to the blood cell wall.
Laboratories
In the laboratory, EDTA 2Na is widely used to inhibit metal ions: In biochemistry and molecular biology, ion depletion is commonly used to inactivate metal-dependent enzymes, either as a measure of their reaction or for Damage suppression of DNA, proteins, and polysaccharides.
In analytical chemistry, EDTA 2 sodium is used in complexometric titration and water hardness analysis or as a coating material to separate metal ions that interfere with the analysis.
Agriculture
Industries use of EDTA to produce fertilizer for plants. These fertilizers can increase the growth of plants.
Is Disodium EDTA safe?
It is safe to use EDTA-2Na; however, you should not release it into the environment because this substance can reduce the absorption of heavy metals by entering the soil. On the other hand, if it goes through the groundwater, it is able to cause pollution of these resources. It is also dangerous to keep it away from industrial and laboratory environments.
Therefore, you should pay attention to all of the health and safety points while working with this substance. Also it is important to wear appropriate clothes and gloves. Consumption of high concentrations may lead to anaemia, fever, and chills. Inhalation of this substance in high concentrations may cause headaches and dizziness.
- Suitable packages should be used for storing this chemical substance. You should keep it in a cool, dry place away from heat and direct sunlight.
The bottom line
EDTA-2Na is a powerful chelating agent that can be used in several industries, such as cosmetics, food, and textiles. Doctors use this chemical substance in lead poisoning treatment. Some of them believe that disodium EDTA has antioxidant effects. It is safe to use this additive in products, but you should not release it into the environment because it can make the groundwater polluted.
Frequently asked questions
Is disodium EDTA safe for skin?
Yes, FDA reported that it is safe to use it in skincare products.
What is disodium EDTA used for?
EDTA-2Na is widely used as a chelating agent in the cosmetic, food, pharmaceutical, and agricultural industries.
What are the risks of EDTA?
Some people show an allergic reaction to EDTA, so they should check the ingredients before consuming a product.
What are the side effects of EDTA on skin?
If you are allergic to EDTA, skin problems may appear.
What is the difference between Na2 EDTA and EDTA?
There are two types of EDTA: EDTA-2Na and EDTA-4Na. The first one has two sodium cations, while the second one has four.






Tam –
Is disodium EDTA safe for skin?
china chemicals –
In several studies, the Cosmetics Review Board has found that as used in cosmetics products, disodium EDTA is safe.
David –
Is disodium EDTA neutral?
china chemicals –
No, EDTA 2 Na’s pH is 4 to 6.