Description
Calcium Hypochlorite, also known as Perchlorine, and has the chemical formula ca (OCl) 2, is one of the industrial chemicals that has many uses. The most important application, which is very strong, is in disinfecting municipal water.
It is relatively stable and more readily available than sodium hypochlorite, which is used as (liquid bleach). Shanghai Chemex is one of the most reputable manufacturers of this chemical in the world.

Physical and Chemical Properties:
Calcium hypochlorite is usually available in white powder, granules, or pellets. This product dissolves quickly in water and releases oxygen and toxic chlorine gas when heated.
It is not flammable, but can act as an oxidizer for combustible materials and may react explosively with ammonia, amines, or organic sulfides.
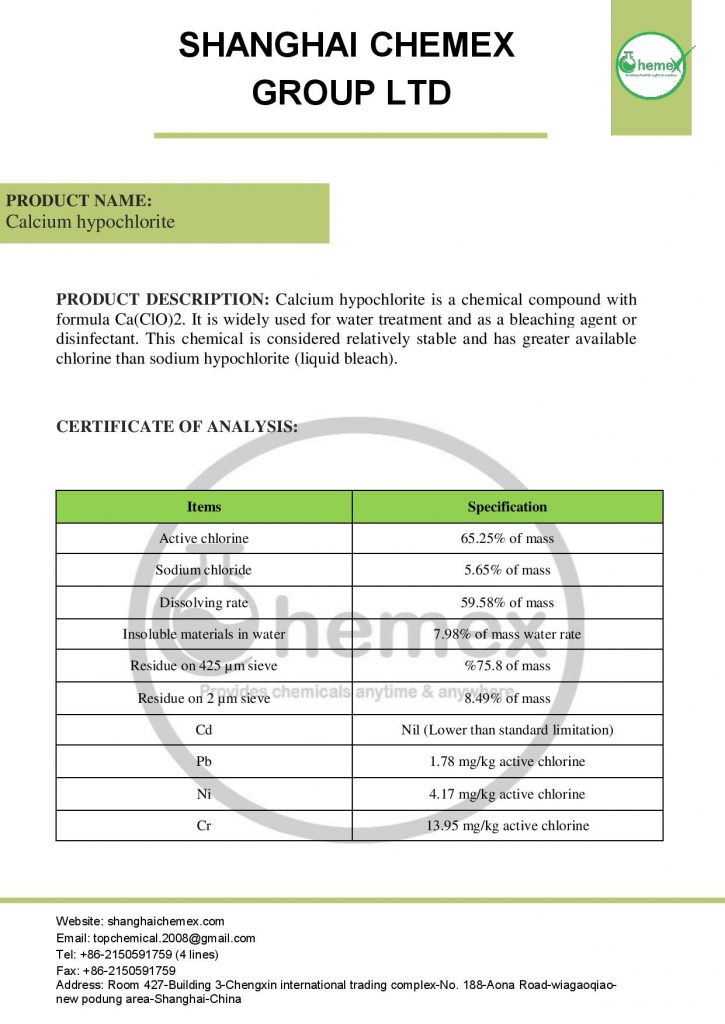
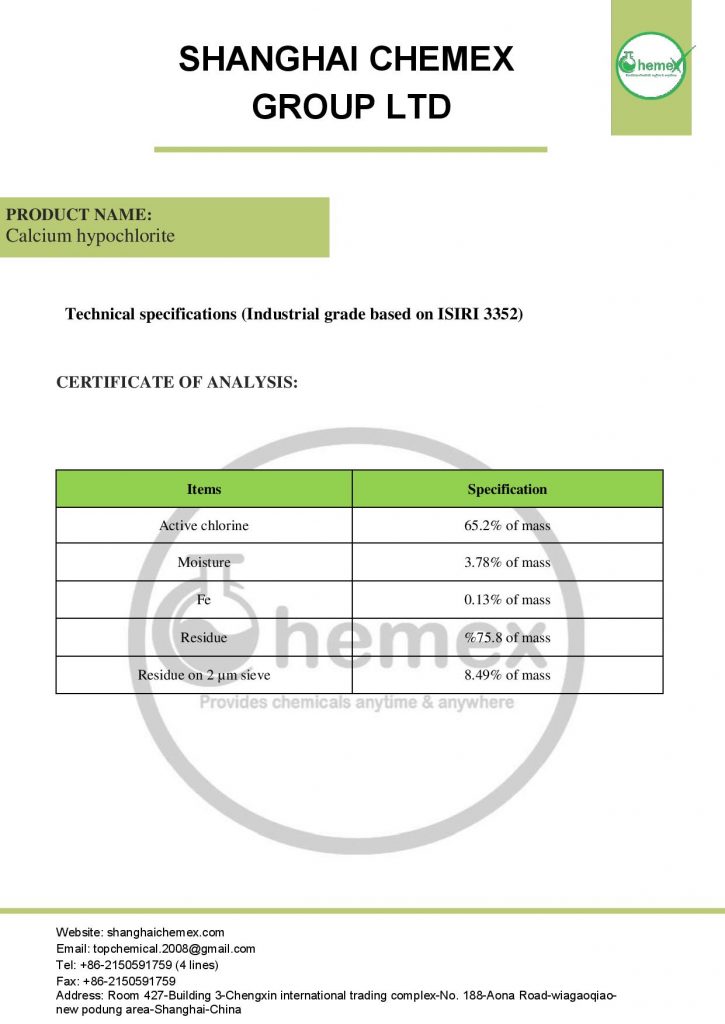
The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | Ca (OCl)2 |
| Molecular Weight(g/mol) | 142.98 |
| Appearance | solid |
| odor | Strong chlorine odor |
| Density (g/cm3) | 2.35 |
| Melting point (° C) | 100 |
| Boiling point (°C) | 175 |
| Water Solubility (g/100 mL at 25 °C) | 21 |
| Other names | Bleaching powder, Hypochlorous acid, calcium salt, Chlorinated lime |
| color | White |
| form | Powder, granules, pellets |
| Chemical Structure Depiction | 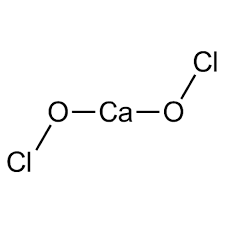 |
Sodium Hypochlorite and Calcium Hypochlorite:
Sodium and calcium hypochlorite, while having a series of similarities, also have differences. For example, both are chlorinated mineral compounds that are commonly used for disinfection. But their differences include more chlorine in calcium hypochlorite. It contains approximately 79% chlorine, which is higher than sodium. Sodium hypochlorite is used to dechlorinate in places where there is a large volume of water and calcium is used in places with less water capacity.
Calcium Hypochlorite Production Process:
Perchlorine is generally produced by two methods, sodium and calcium, which is the most widely used method of sodium today.
The calcium method is based on the chlorination of crushed lime through direct consumption of chlorine gas based on the following reaction:
2Ca (OH)2 + 2Cl2 → Ca (ClO)2 + CaCl2 + 2H2O
The sodium method is based on the reaction of sodium hypochlorite with the product of the calcium method. To remove the undesirable calcium chloride is as follows:
CaCl2 + 2NaClO → 2NaCl + Ca (ClO)2
Calcium Hypochlorite Uses:
The use of calcium hypochlorite is mostly due to its use in drinking water treatment. While this material is used in pool water treatment, production of paper and related textiles, laundries, disinfection of environments and places and surfaces, production of home bleach, and various items of organic chemistry. In the following, we will discuss how this combination works in each of these uses:
Water treatment:
Chlorine in all its forms (gas, liquid and solid), is the most important disinfectant for drinking water and swimming pools.
The mechanism of action of perchlorine is through its small, specific molecules that can pass through the cell membrane of bacteria. These molecules can kill germs by affecting the oxidation of the cells of microorganisms and inactivating their enzymes.
Disinfection of vegetables:
Calcium hypochlorite is used in the disinfection of vegetables and other plant products, which is not a scientific and correct thing to do. Perchlorine is classified in a large family of hazardous chemicals and can be pathogenic to humans in the long run if absorbed by vegetables and not thoroughly washed.

Laundry:
The advantage of this material over washing powder is its low-temperature washable property, which preserves both the fibers and color of the fabric and saves energy.
Paper production:
Chlorine bleach is also used in the paper industry. Calcium hypochlorite is used to discolor waste paper and remove the ink.
organic chemistry:
Calcium hypochlorite is a common oxidant in organic chemistry. For example, it is used to produce glycols, carboxylic acids, keto acids, and the toxic gas chloroform.
Similar product: Glutaraldehyde
Buy Calcium Hypochlorite:
Shanghai Chemex is a manufacturer of chemicals and a supplier of all kinds of chemical goods. You can contact our experts to find out the price and purchase of this product.
Safety Information:
In case of contact of calcium hypochlorite with any part of the body, it can cause various complications such as allergies or other physical problems, and in all these cases, only water should be used for washing. In case of inhalation, ingestion, or physical contact with perchlorine accidentally, large amounts of water should be consumed immediately and the casualty taken to the nearest hospital.
Packing and Storage:
Due to its high corrosive and oxidizing power, this material should be stored in a dry, cool place, away from direct sunlight, chemicals, incendiary substances, oils, fats, petroleum derivatives, and Burning materials.






Reviews
There are no reviews yet.