Description
Sodium bisulfate is the name of an inorganic acidic salt that is available in powder or white crystal form, its smell is similar to sulfur. This chemical is also known by other names such as sour rock or sodium hydrogen sulfate. This inorganic compound has a variety of uses and can be widely used in a variety of applications to reduce acidity. The two most common types of this inorganic salt are dry or monohydrate. Shanghai Chemex is one of the most reputable manufacturers of this chemical in the world.

Physical and Chemical Properties of Sodium Bisulfate:
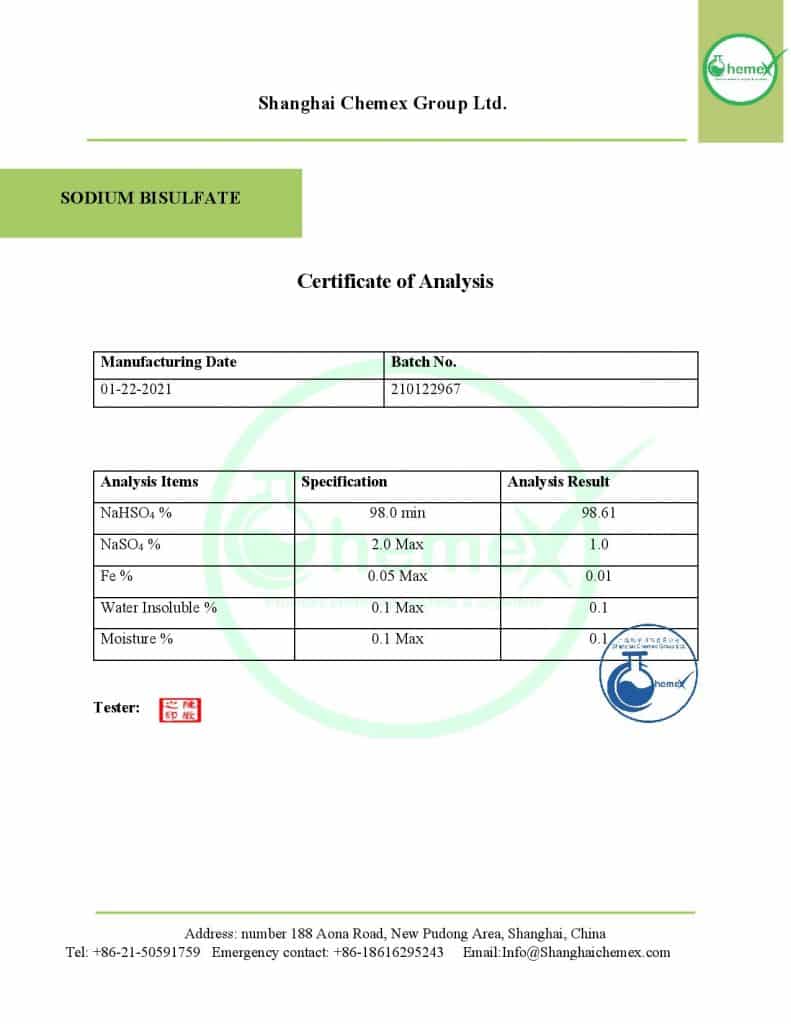
As mentioned, sour rock is a type of salt of the sulfuric acid family and it can be called hydrogen sulfate; The most important physical and chemical properties of this material can be summarized in the following table:
| Name | Sodium bisulfate |
| molecular weight (g/mol) | 120.06 |
| Density (g/cm³) | 2.74 |
| Boiling point (°C) | 315 |
| Solubility in water | Soluble |
| PH | 1 |
Synthesis and Production of Sodium Bisulfate:
generally, there are two methods for the synthesis of sodium bisulfate, we will mention them below:
- In the first method, it is done by two substances, sulfuric acid, and sodium hydroxide. By performing a reaction between these two substances, sour rock and some water are formed.
- In the second method, sodium chloride reacts with sulfuric acid, and the desired product is formed with hydrochloric acid. This salt is formed in different reactions, it can be produced by the reaction of other salts with sulfuric acid.
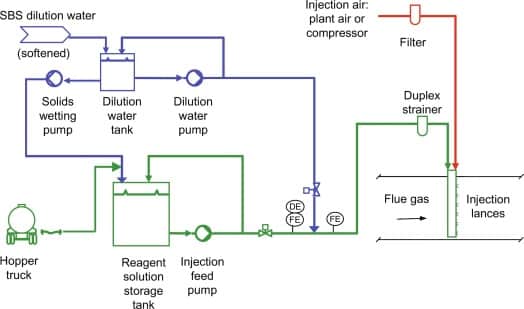
Sodium Bisulfate Uses:
The most important uses of this material include the following:
- sour rock can be used as an activator in polymerization processes.
- In the food industry, a food additive can delay food spoilage.
- This chemical is also used in the chemical industry in a reducing role and for the purification and separation of ketones.
- This salt is generally used in the leather industry to degrease the skin.
- In the water treatment industry, it can be used to dechlorinate water.
- This inorganic substance can be used as a source of sulfur dioxide production.
- It is also used as an additive in the production of cleaning and disinfecting products.
Sodium Bisulfate Hazards:
- In some cases, this substance can cause severe irritation or irritation of the skin. In case of contact with this product with the skin, it should be washed with plenty of water for at least fifteen minutes. Clothes containing contaminants should then be removed and washed again.
- If eyes come in contact with sour rock, they should be rinsed with plenty of water first and then medical warnings should be consulted.
- If you inhale sour rock, you should rinse your mouth and give one or two glasses of water to the person, and then refer to safety considerations.
Transporting and Storage:
It is better to store this material in a dry and cool place away from heat. It is also better to have a storage room for this material with proper ventilation.






Reviews
There are no reviews yet.