Description
Sodium dichromate is a solid red or orange, an odorless crystal that can absorb moisture and has a high solubility in water. Sodium chromate and dichromate are the most important compounds of chromium, which are mostly used to produce chromic acids and chromium pigments used in leather tanning and corrosion control. The industrial uses of this material are very wide, which we will discuss in the following. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
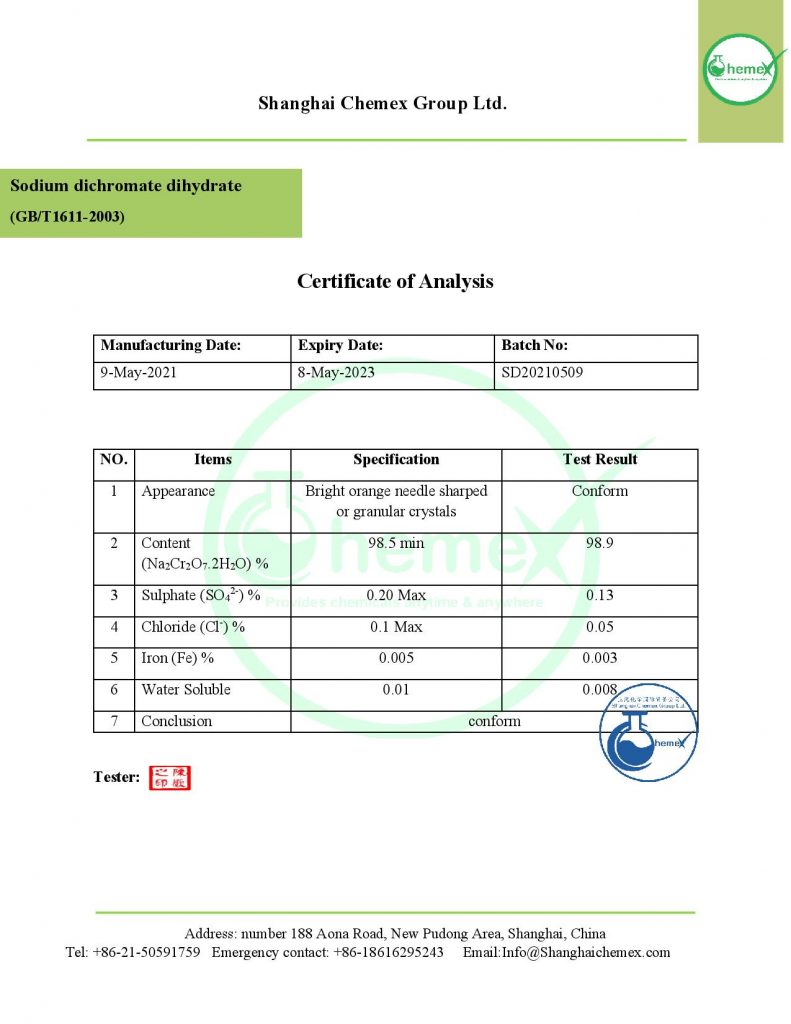
Sodium dichromate is a mineral, orange, odorless, and soluble in methanol and ethanol, commonly used as a dihydrate salt. Almost all chromium compounds are made from this salt. This is an inorganic compound that is more resistant and harder than metal oxidants such as titanium or iron, it can be used in harsh environmental conditions such as high temperatures; The most important physical and chemical properties of this product can be summarized in the following table:
| Chemical formula | Na2Cr2O7 |
| Molecular Weight(g/mol) | 261.97 |
| Appearance | solid |
| odor | Odorless |
| Density (g/cm3) | 2.52 |
| Melting point (° C) | 356.7 |
| Boiling point (° C) | 400 |
| Solubility | soluble in water, methanol, ethanol |
| Other names | Bichromate of soda, Disodium dichromate, |
| color | bright orange |
| form | crystalline |
| Chemical Structure Depiction | 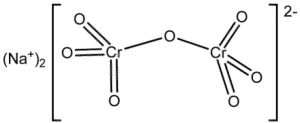 |
Sodium Dichromate and Potassium Dichromate:
Sodium dichromate and potassium dichromate are very similar in reactivity and appearance. The main advantage of sodium dichromate over potassium dichromate is that it has more solubility in water and polar solvents such as acetic acid.
Synthesis and Production Process of Sodium Dichromate:
Sodium dichromate is generally obtained from minerals that have chromium oxide in their structure, which is described in the following stages of its production:
The ore is first reacted with a mixture of sodium carbonate and air at very high temperatures, resulting in carbon dioxide and sodium chromate, then dissolved in relatively warm water, at which point other ore compounds such as Aluminum and iron compounds are insoluble in water and precipitate and separate. Acidification of the resulting solution with sulfuric acid or carbon dioxide gives sodium dichromate, which is then separated by crystallization as dihydrate.
Sodium Dichromate Uses:
Mainly dichromate and chromate salts are used in the role of an oxidizing agent. In the production of organic chemical compounds, benzyl and allyl bonds are used as oxidizing agents and can oxidize some alcohols to ketones.
The use of this salt is very broad, the most important of which are as follows:
- sodium dichromate is widely used in metal plating and prevents corrosion of metals.
- This material is present in a variety of coolers, including alcohols and water cooling systems, due to the formation of a protective membrane on metal surfaces.
- This organic chemical is Used to produce mineral chromate pigments, which contain a very wide range of stable dyes.

- sodium dichromate is a useful material in the production process of colored glass and ceramic glasses. like Chrome oxide
- This substance is added as an additive to acidic dyes to increase the speed and improve the coloring properties.
- In the synthesis of organic matter, this salt acts as an oxidant in the reaction in the presence of sulfuric acid.
Sodium Dichromate Price:
You can contact our experts in Shanghai Chemex to find out the price of this product and other chemical products.
Safety:
This product is corrosive and of course, working with it and contact with it causes allergies, irritation, and irritation in some parts of the body. Here we are going to introduce ways to minimize the risks of contact with this chemical:
- Eye Contact: In case of contact with the eyes, immediately flush the eyes with plenty of water.
- Skin Contact: Remove this material from the skin with plenty of soap and water.
- Inhalation: Remove to fresh air immediately. If not breathing, give artificial respiration.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Therefore, when working with this chemical compound, observe general safety and hygiene and use safety gloves and special glasses.
Transporting and Storage:
Store this material in a cool, dry environment with a well-ventilated area away from heat.






Reviews
There are no reviews yet.