Description
Mono ethylene glycol is an organic compound with the chemical formula (CH2OH)2, which is mainly used as a raw material in the manufacture of polyester fibers and in antifreeze formulations. MEG was first produced by a French chemist. Over time, due to the desirable properties of this solvent, such as high strength and hydrophobicity, its applications have expanded greatly. The Shanghai Chemex company provides this chemical with the best quality for you; In the following, we will learn more about the physical and chemical properties as well as the wide applications of this material.
How to buy mono ethylene glycol (MEG)
If you would like to know any further information about mono ethylene glycol`s price or buying this chemical item, our team at Shanghai Chemex will be delighted to help you.
Physical and Chemical Properties:
Pure mono ethylene glycol formula is (CH2OH)2. It is a toxic, clear, colorless liquid with a mild, sweet-tasting, syrupy concentration. This compound is miscible with water and aromatic and aliphatic hydrocarbons, has good heat transfer ability, and is usually used by systems that require a constant temperature. The most important physical and chemical properties of this organic compound can be summarized in the following table:
| Name | MEG |
| Chemical formula | (CH2OH)2 |
| Molecular weight (g/Mol) | 62.07 |
| Appearance | colorless liquid |
| odor | Odorless |
| Boiling point (°C) | 197 |
| Melting point (°C) | -12.9 |
| PH | 5.5-8 |
| Solubility | Soluble in most organic solvents |
| Mono ethylene glycol structure | 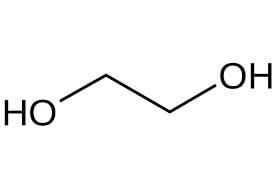 |
Let me add one more point about this material properties. Mono ethylene glycol density is 1.11 g/cm³.
The Production Process of MEG
Mono ethylene glycol (MEG) is produced from ethylene by an intermediate known as ethylene oxide. In this process, the intermediate (ethylene oxide) and water react together, and ethylene glycols, including MEG, DEG, and TEG (triethylene glycol), are produced. One of the interesting points about this reaction is that it can be performed in environments with different pH levels, but to achieve higher efficiencies, it is recommended to perform the reaction in an acidic or neutral environment with excessive amounts of water.
Production stages
Ethylene and oxidizing agents are first introduced into a reactor, the product of this reaction is ethylene oxide. This reaction is considered exothermic and takes place in the gas phase with silver catalysts. During the process, water vapor is produced by the heat of the reaction. The product obtained from the reactor is sent to the ethylene oxide (EO) adsorption unit. Some of the gas vapor is returned to the reactor and the rest is sent to the carbon dioxide removal unit, which includes an adsorbent and a stripper. In this unit, carbon dioxide is separated for use in the production of ethylene carbonate. After this step, the diluted vapor of ethylene oxide is removed from the adsorbent and concentrated during the process. Finally, pure ethylene oxide is hydrolyzed to produce mono ethylene glycol and carbon dioxide, a reaction that usually takes place in the liquid phase.
(MEG) Mono Ethylene Glycol Uses
Among the important and industrial applications of mono ethylene glycol (MEG) are the following:
Manufacturing polymers
MEG can be used as a raw material for the manufacturing of polymers such as polyesters and polyethylene terephthalate. For example, this material plays an important role in producing a variety of polymer resins, polyesters for rubber and production of shoe rubber.
Anti-freezing agent
MEG has a low freezing point, so this material is used as a cooling material and facilitates heat transfer in air conditioning systems. It can also prevent water from freezing inside chillers and cooling equipment in industries.

This special property makes it a good choice to be the main component of car antifreeze formulations.
Mono Ethylene Glycol as a Coolant and Antifreeze
As mentioned, one of the most important applications of ethylene glycol is its use in the production of antifreeze. The chemical dissolution of this compound in water causes the hydrogen bond of the water to be overshadowed and fused, causing the freezing point. To produce antifreeze based on mono ethylene glycol, a certain amount of this substance is mixed with water and a suitable corrosion inhibitor and repellent. It is better not to use more than 70% of MEG, using corrosion inhibitors in this formulation is necessary. (like Propylene glycol)
Oil and gas industries
You might have heard about MEG in the oil and gas industries. In fact, this material is used As a dehydrating agent to remove water molecules from natural gas.
Other applications
It is used in the ink industry to reduce viscosity, cost, and volatility. Moreover, MEG has a special role in the manufacture of fibreglass and in synthesizing some carbonyl organic compounds.
 Industries also use it in producing leather and as a humectants for paper.
Industries also use it in producing leather and as a humectants for paper.
Mono ethylene glycol MSDS
In this part we are going to discuss about safety, hazard and poisoning of MEG:
This compound is classified in the list of toxic and dangerous substances for human health, and swallowing, skin and eye contact, and breathing should be avoided. In case of contact with skin and eyes, rinse immediately with plenty of running water and drink plenty of fluids if swallowed.
“Ethylene glycol was first synthesized in 1859; however, it did not become a public health concern until after World War II. In fact, the first published series of deaths from ethylene glycol consumption involved 18 soldiers who drank antifreeze as a substitute for ethanol.”
Sciencedirect
The major danger of mono ethylene glycol is its environmental threat. Because of its liquid nature, it easily penetrates the soil and can lead to groundwater pollution. However, due to the high toxicity of this substance, its use is prohibited in the following cases:
- Water consumption equipment
- Formulation of food
- as antifreeze in water tanks
- as an antifreeze in fire extinguishing systems
Packing and Storage
Use a cool and dry place to store this chemical away from heat and direct sunlight.
Frequently asked questions
What is mono ethylene glycol`s viscosity?
MEG viscosity is 18.376 cP at 20.0 °C. In fact, this chemical has a dynamic viscosity.
What is mono ethylene glycol`s price today?
You can contact us via the numbers below this page. Our team at Shanghai Chemex will give you the information you need about this chemical item.
What is mono ethylene glycol used for?
MEG is widely used as an antifreezing agent. Additionally, we can use mono ethylene glycol in the oil and gas industry to remove water from natural gas.
What is the difference between mono ethylene glycol and ethylene glycol?
There is no difference between MEG and ethylene glycol. Actually, MEG is the other name for ethylene glycol.
What is MEG in coolant?
It is widely used in car or boat engines cooling systems as an antifreezing agent. This chemical is so common for this application, especially in cold countries.







Reviews
There are no reviews yet.