Formic acid, also known as ant ink, is the simplest type of acid among the large group of carboxylic acids. This chemical is made naturally from insect bites such as ants and bees. In recent decades, with the advancement of science and sciences such as chemistry, scientists have been able to synthesize this chemical in the laboratory.
This is an organic acid that was first obtained in 1671 by a naturalist named John Ray from the distillation of a group of ants. But years later, this acid was also obtained by Joseph Gilossack of hydrocyanic synthesis. Another laboratory synthesis was obtained in 1855 from carbon monoxide. This method is currently the best and easiest way to make formic acid. There are other industrial methods for preparing this acid that is produced from products such as methanol, oxalic acid, and acetic acid.
Industrial Preparation of Formic Acid:
1. Formic Acid and Methanol
Methanol belongs to the category of alcohols in the classification of chemicals. It is a colorless, volatile, flammable, and toxic liquid with the chemical formula CH3OH. It is produced from the destructive distillation of wood and mainly from carbon monoxide and hydrogen. Its main applications are in the synthesis of organic acids such as formic acid, and acetic acid as a solvent.
In this method, formic acid is produced during two stages of methanol carbonylation and production and hydrolysis of methyl formate. About 65% of the producers of this acid in the world use this method.
Step 1: Methanol Carbonylation and Methyl Formate Production:
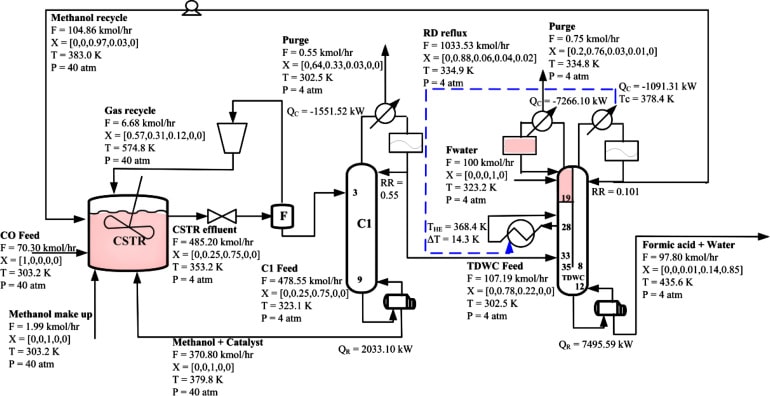
At this stage, in order to purify the feed, CO gas passes through the absorber substrate V-101 and V-102 and the moisture in it is completely absorbed, then it is increased to 65 atmospheres by the K-101 compressor. And transferred to the R-101 carbonation stirrer reactor.
After mixing with the sodium catalyst, the methanol feed is mixed with the return methanol stream and sent to the reactor. This return current is cooled by the E-103 converter. The output current from the reactor contains compounds such as methyl formate, methanol, and carbon monoxide, which are sent to the C-101 distillation column to separate the methyl formate product. It should be noted that the return flow from the hydrolysis section of methyl formate is also sent to the tower.
The operating pressure of the tower is atmospheric and out of the upper part of the tower, light compounds of methyl formate and Carbon monoxide are released. After cooling in two stages by the converter and condenserE104 -E105, non-condensable carbon monoxide gas is sent to the atmosphere Reflux is returned to the tower in a certain ratio and the rest is transferred to the next stage. Liquid methanol along with the catalyst is removed from the bottom of the tower and returned to the carbonylation reactor.
CH3OH + CO → HCOOCH3
Step 2: Hydrolysis of Methyl Formate and Production of Formic Acid:
The methyl formate output from the top of the tower will enter the R-202 hydrolysis reactor after heating in the E-201 converter along with the return water flow from the process. The liquid flow from the reactor mainly consists of formic acid, methyl formate, and water, which is sent to the C-201 distillation tower to purify the product.
The operating pressure of the tower is atmospheric. Light compounds such as methyl formate and wet methanol are released from the top of the tower and after passing through the E-203 condenser, part of it is returned to the column by reflux flow and the rest to the drying column C-202 will be sent. In the drying column, a mixture of methanol and methyl formate is removed from the top of the column and after condensation is sent to the C-101 distillation column by the E-204 converter for separation. The effluent from the bottom of the azeotropic column is 77% formic acid and water, which is sent to column C-301 for concentration. In this column, under operating pressure of 6.2 atmospheres, a solution of 85% formic acid is released from the bottom of the tower and the output water is sent from the top of the tower to the R-201 hydrolysis reactor.
HCOOCH3 + H2O →HCOOH + CH3OH
2.Formic Acid and Acetic Acid
Formic acid and acetic acid are both organic molecules that can be classified as carboxylic acids due to the presence of the carboxyl group. Therefore, both compounds are acidic compounds. The main difference between formic acid and acetic acid is that formic acid consists of a carboxyl group attached to a hydrogen atom, while acetic acid consists of a methyl group attached to a carboxyl group.
Formic acid is produced as a by-product in the oxidation of the liquid phase of hydrocarbons to acetic acid. In the United States, butane is used as a hydrocarbon, producing approximately 50 kilograms of formic acid per tonne of acetic acid. In Europe, naphtha oxidation is preferred and up to 250 kg of this acid is obtained from each ton of acetic acid.
Licensed companies Celanese, BP, and Daicel produce formic acid as a by-product in the production of acetic acid by normal oxidation of butane. It is estimated that about 15% of factories producing this acid use this method.
3.Formic Acid and Oxalic Acid
Oxalic acid is a colorless organic solid crystalline compound that forms a colorless solution in water. This acid is classified as the simplest dicarboxylic acid. Its applications can be mentioned as a factor in the production of formic acid.
Formic acid can be obtained in the laboratory by heating oxalic acid in anhydrous glycerol and extracting the product by steam distillation.






4 Responses
Is formaldehyde oxidized to formic acid?
The main product of formaldehyde oxidation is formic acid. The by-products of this process are carbon oxide and methyl formate.
Sir,
I will be very grateful to you if you could let me have the name telephone and E-mail to contact as the licensor
for the formic acid process technology. Eastman Chemical Company, BASF, etc., will be thankfully recieved.
Eliahu Rosenthal
Hi,
Ways to communicate with our sales unit expert:
Tel: +86-2150591759
WhatsApp:+86-16601896001
Email: info@shanghaichemex.com