Description
Salicylic acid is a beta hydroxy acid known chemically as mono hydroxybenzoic acid. This product is synthesized as an organic acid from the amino acid phenylalanine, and plants can also release significant amounts of this acid. Salicylic acid has many medicinal properties and is used in the pharmaceutical industry. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
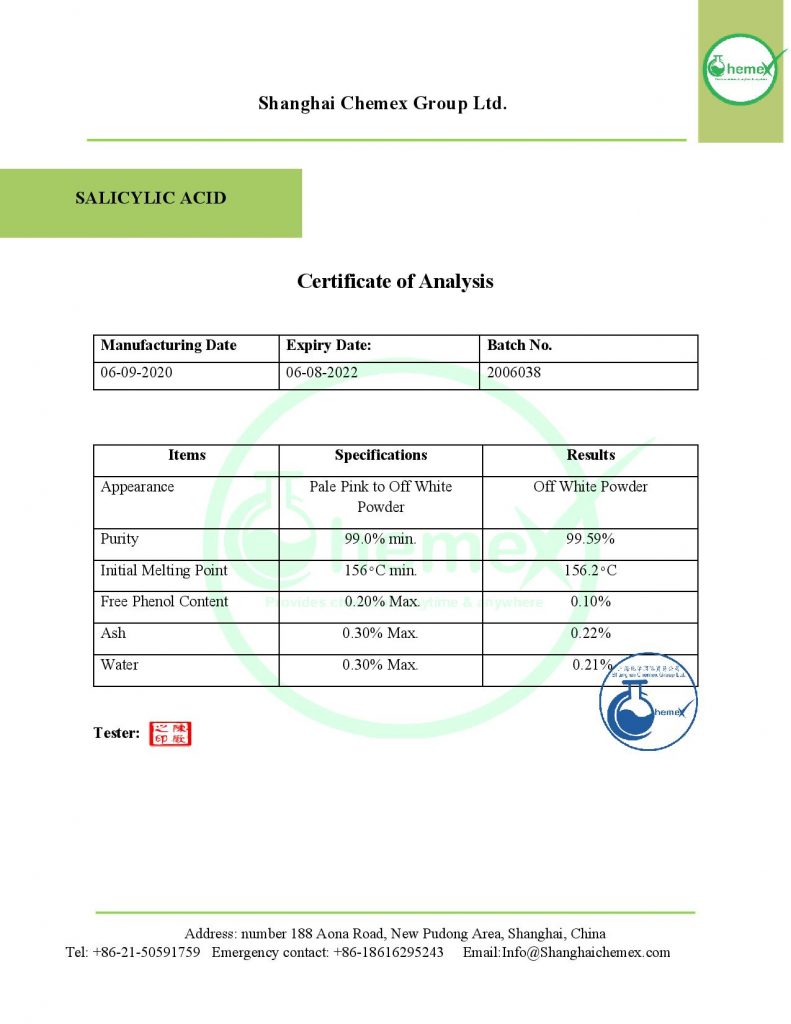
Salicylic acid with the chemical formula C7H6O3 is a white to yellowish-brown odorless solid. This compound has little solubility in water and produces irritating and smoky vapors with a very unpleasant odor when decomposed; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | C7H6O3 |
| Molecular Weight (g/mol) | 138.122 |
| Appearance | Solid |
| Density (g/cm3) | 1.443 |
| odor | Odorless |
| Taste | Acrid taste |
| Melting point (° C) | 158.6 |
| Boiling point (° C) | 200 |
| Solubility | Soluble in water, ether, CCl4, benzene, propanol, acetone, ethanol, oil of turpentine, toluene |
| Color | White, Colorless |
| Form | crystalline powder |
| Chemical Structure Depiction | 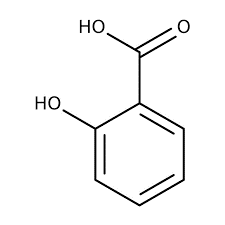 |
Formula and Structure of Salicylic Acid:
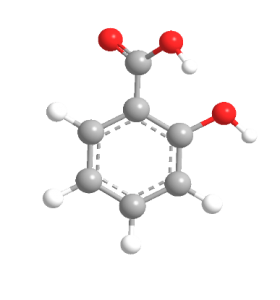
The structural formula of this compound is C6H4 (OH) COOH, which consists of a hydroxyl group attached to the benzene ring in the ortho position with respect to the carboxylic acid functional group. The carbon atoms in the benzene ring of this material are hybridized. Salicylic acid also forms an intramolecular hydrogen bond.
Synthesis of Salicylic Acid:
To produce salicylic acid, sodium phenolate first reacts with carbon dioxide in the presence of sodium hydroxide at a pressure of 100 atmospheres at temperatures above 116 ° C.
This will produce sodium salicylate. In the next step, the obtained substance is acidified with sulfuric acid and finally, salicylic acid is produced.
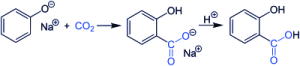
It can also be prepared by hydrolyzing aspirin or methyl salicylate with the help of strong acid or base. It decomposes to phenol and carbon dioxide at a temperature of 200-230 ° C.
C6H4OH(CO2H) → C6H5OH + CO2
Salicylic Acid Uses:
- Salicylic acid as a plant hormone is widely used in the synthesis of organic matter.
- Used to make aspirin and many skin medications.
- This acid is used as a food preservative, antibacterial and antiseptic.
Salicylic Acid as a Skin Exfoliator:
This compound is commonly used as a skin exfoliating drug. They are also used to treat warts, acne, psoriasis, acne, dandruff, and moles.
The mechanism of action:
Salicylic acid penetrates the surface of the skin and dissolves the factor that holds cells together. This kills dead skin cells that clog pores. Therefore, it reduces the appearance of blackheads and prevents the appearance of pimples. It is also fat-soluble and can work in oily environments, so it is very effective in reducing skin oil. In addition, salicylic acid is antibacterial and anti-inflammatory, thus helping to soothe the redness and swelling of existing blemishes as well as prevent the formation of new blemishes. like Benzoyl peroxide
Buy Salicylic Acid:
Contact our experts in shanghai Chemex to buy salicylic acid in both pharmaceutical and industrial grades and to know its selling price.
Safety Information:
This compound, like other chemicals, in addition to its benefits and applications, also has disadvantages. Therefore, it is necessary to observe safety tips when working with it, including wearing a cape and gloves and using glasses and masks. Contact of this substance with the eye causes severe eye irritation. May cause corneal damage. Skin contact can also cause irritation and possible burns, especially if the skin is wet or damp. If absorbed, it may cause symptoms similar to those of consumption. It May cause skin rash and pimples. Ingestion of this substance stimulates the gastrointestinal tract and other symptoms such as nausea, vomiting, and diarrhea. Inhalation of this substance also stimulates the membrane of the respiratory tract.


First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
Keep the container tightly closed in a dry, cool, well-ventilated place away from incompatible materials.







Reviews
There are no reviews yet.