Description
Benzene is a chemical substance with the molecular formula C6H6, a widely used and at the same time dangerous substance in industry and laboratories. Due to its peculiar structure and possible applications, this compound is known as one of the most important and at the same time most widely used aromatic compounds. From a practical point of view, benzene is one of the most important raw materials for the production of other chemicals in various industries. These include construction, electrical and electronic industry, textile industry, and automotive industry. Shanghai Chemex is one of the most renowned suppliers of this chemical in the world.
Physical and Chemical Properties:
Benzene is in fact a colorless, toxic, carcinogenic, yet an aromatic liquid with a high evaporation rate that is also extremely flammable; The most important physical and chemical properties of this aromatic compound can be summarized in the table below:
| Name | Benzene |
| Molecular weight (g/Mol) | 78.11 |
| Odor | Aromatic odor |
| Viscosity (μPa s) | 228.4 |
| Melting point (°C) | 5.5 |
| Boiling point (°C) | 80.1 |
| PH | 1-3.5 |
| Solubility in water | Completely soluble |
| Color | Colorless to light yellow |
| Form | liquid |
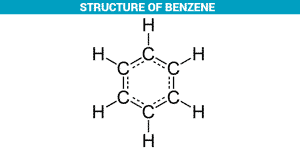
Benzene Structure:
According to the molecular formula of benzene, different structures are imagined for this molecule. Organic chemists in the nineteenth century had various speculations about the structure of this molecule. Some of them were rejected due to the number of isomeric products derived from benzene, and others were accepted until the original structure of benzene was discovered by Friedrich August Kokole in 1865.
Benzene is a ring molecule in which the six carbon atoms form a regular hexagonal structure, each attached to a hydrogen atom. He stated that covalent bonds between carbon atoms are one-way, simple, and dual. Of course, he believed that the place of simple and double bonds changes quickly, and this makes the length of carbon bonds the same.
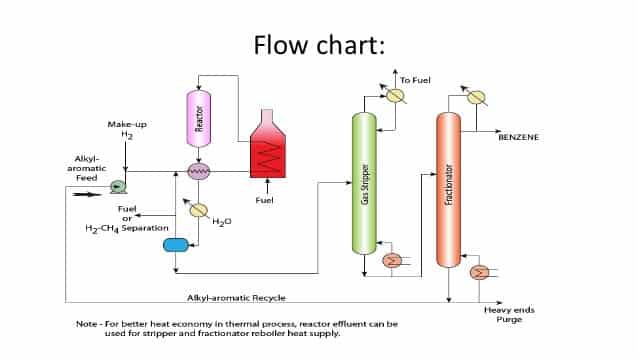
Benzene Production Process:
There are different methods for producing benzene. In the first method, in the presence of platinum chloride and at a temperature of 60 to 200 ° C, a group of hydrocarbons reacts with hydrogen and then this organically is produced by processes such as distillation and filtration. The second method is steam cracking, which is a good way to produce ethylene and other alkenes from hydrocarbons. Benzene is also a product produced from the pyrolysis reaction of diesel.
Benzene Uses?
Due to its unique properties, this aromatic compound has found many applications in industry or even in our daily lives, of course, since the dangers of this substance were reported, its use was limited or alternatives such as acetone or toluene were used. Here are the most important uses of this solvent:
- Benzene is used as a solvent and in the production of chemicals such as phenol, nitrobenzene, and cyclohexane.
- This organic compound is widely used in the printing industry as a solvent to regulate the viscosity of the ink.
- This organic compound is used in the production processes of polymers, especially in the rubber industry.

- Benzene is also used as an adhesive in the shoe industry and the production of various types of shoes.
- It is used in the paint and resin industry and the production of plastics and lubricants. like Butyl DI glycol
- This aromatic compound can play a good role as a cleaner and degreaser in the industry, especially for cleaning and degreasing metals, hydraulic systems, or fuel systems.
Buy benzene:
To buy benzene and find out the price of this product through the communication channels available on the site, you can contact our experts in Shanghai Chemex.
Safety Information:
Benzene is known to be a toxic and carcinogenic compound, so any use of this chemical should be done under a hood and with laboratory gloves. This chemical liquid was used as a solvent in laboratories in the past, but after scientists realized that it was toxic and carcinogenic, they tried to use similar solvents such as acetone. This chemical is so dangerous that long-term use can have devastating effects on the tissues that make up blood cells, especially bone marrow cells. Other dangers of using benzene include:
- Disorders of the human respiratory system
- Decreased ability of the immune system
- Chronic amnesia or Alzheimer’s
- sterility
- Decreased hematopoiesis
Packing and Storage:
Barrels containing this organic chemical should be stored in a cool, dry place away from heat or direct sunlight.






Reviews
There are no reviews yet.