Description
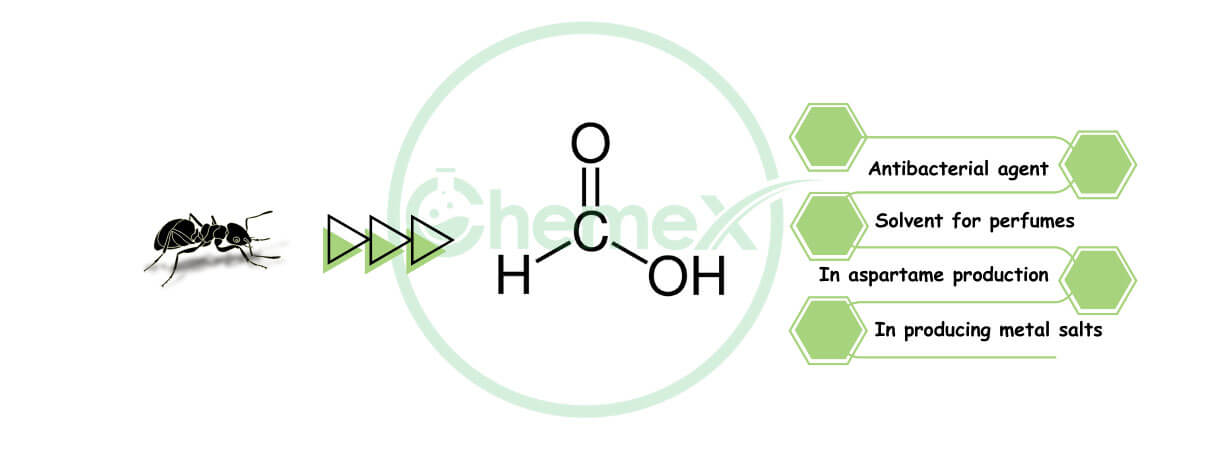
Formic acid (FA) is also known as Methanoic acid, and it falls into the category of carboxylic acids. It is a strong organic acid that many people recognize as “ant ink” because formic acid is found in the bites of many insects, such as bees and ants. FA is available in both liquid and solid forms, and nowadays it is widely used in the textile, food, and animal industries. In this article, we are going to discuss the two main questions: what is formic acid? And why do we use it in many industries?
Where to buy formic acid?
If you would like to know any further information about formic acid`s price or how to buy this item, our team at Shanghai Chemex will be delighted to help you with that.
Physical and Chemical Properties
Formic acid is a colorless, corrosive, and volatile liquid with a pungent odor. This acid dissolves in water and in ether, acetone, ethyl acetate and can be partially combined with benzene, toluene, xylenes. It is extremely stable at room temperature and has low flammable properties.
The most important physical and chemical properties of this FA are summarized in the following table:
| Chemical formula | CH2O2 |
| Molecular Weight (g/mol) | 46.025 |
| Appearance | Colorless liquid with pungent odor |
| Density (g/cm3) | 1.220 |
| CAS number | 64-18-6 |
| taste | sour |
| Boiling point | 101°C |
| Melting point | 8.3°C |
| Flash point | 69 °C |
The formic acid pH is about 2–3. It is capable of being decomposed by living organisms in nature.
What is the Structural Formula?
Formic acid’s structure with the chemical formula HCOOH is such that a hydrogen atom is attached to a carboxylic group.
“Formic acid is more easily ionized than acetic acid. Therefore, formic acid is expected to be more corrosive than acetic acid. Indeed, formic acid attacks many common metals and alloys.”
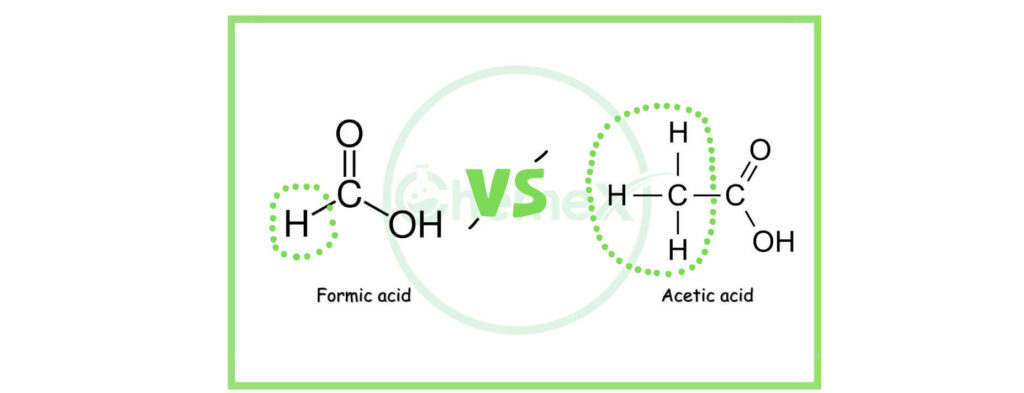
It is similar to the structure of acetic acid. The difference between formic acid and acetic acid is that in acetic acid, the carboxyl group is attached to the methyl group, whereas in formic acid, it is attached to hydrogen atoms.
Formic acid Production Process:
Many years ago, industries produced formic acid from CO2 in the presence of catalysts such as Ru complexes. This reaction took place in the mixture of CO2, tertiary amine, and hydrogen in alcohol, but it had so many problems. For example, the catalysts were so expensive. However, nowadays it is industrially manufactured from methanol.
One of the industrial methods for producing formic acid happens in two phases: In the first step, the carbonylation of methanol occures, and in the second step, the hydrolysis of methyl formate takes place.
CH3OH+CO→HCOOMe
HCOOMe+H2O→HCOOH+CH3OH
The other way of producing FA is as a by-product of acetic acid. However, the amount produced is not enough to meet industrial demand. In 2012, 621,000 t/a of FA were produced.
Formic Acid Uses:
FA is widely used in several industries. in this part we are going to explain some of its applications:
Animal feeds
In Europe, this chemical is used as a preservative and antibacterial agent in animal feed. For example, this substance is used to prevent spoilage of winter food in livestock in large livestock complexes (Like Calcium propionate)
Bees
FA is usually used as a fumigant to kill the mites, which are able to attack the bees.
Leather Production
The second most widely used formic acid industry is the tanning and leather industries. In the process of leather production, the environment is strongly played, which is very unsuitable for leather production, so to reduce the pH of leather, sulfuric acid, and formic acid are added. Formic acid lowers the pH of the leather and penetrates homogeneously into the leather. If this is not done, many unwanted substances such as chromium sulfate will seep into the leather. Also in leather dyeing, this method helps to transfer the color to all parts of the leather.
Metal salts
Industrial uses use it to make metal salts such as nickel, cadmium, and potassium formate.
Producing artificial sweeteners
It has a large role in producing artificial sweetening agents such as aspartame.
Other applications
- In the leather industry and tanning
- In the synthesis of drugs such as insulin and caffeine
- In dyeing and finishing textiles
- As a coagulant in rubber production
- To produce synthetic perfumes
- As a mobile phase in high-performance HPLC liquid chromatography
- As a solvent for perfumes
Safety Information:
Formic acid is dangerous through inhalation, skin contact, or swallowing and can cause corrosion to all tissues in the body. Therefore, observing the safety of working with this material is very important.



First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center
Handling and Storage:
Formic acid is a strong reducing agent and is likely to react against strong oxidizing agents or strong bases. This compound is sensitive to heat and moisture and is likely to decompose in the presence of water and carbon dioxide, releasing irritating gases and flammable vapors. Therefore, this product should be stored in a strong container in a cool, dry, and well-ventilated place away from heat or ignition sources.
Frequently asked questions
What are formic acid used for?
It is used as an antiseptic and antibacterial agent in livestock and poultry feed and also to produce synthetic perfumes. Industries use formic acid in the textile industry.
Is formic acid harmful to humans?
Using this chemical without paying attention to the MSDS is dangerous. This compound is sensitive to heat and moisture and is likely to decompose in the presence of water and carbon dioxide, so you should keep it away from them.
Is formic acid soluble in water?
Is formic acid explosive?
Is formic acid painful?
If the skin exposed to this acid it could cause painful irritations. It can also cause corrosion to all tissues in the body.






linda –
What neutralizes formic acid?
china chemicals –
Baking soda (NaHCO3) is commonly used to neutralize acids, including formic acid. If you were to spill some formic acid solution on your skin or on the floor, you would want to neutralize it with a thick paste of sodium bicarbonate in water.
radan –
How much formic acid is toxic?
china chemicals –
The threshold value for exposure to formic acid in the air is 5 ppm or 9 mg / m3