Description
Hydrofluoric acid is an inorganic chemical compound with the chemical formula HF that is available in both anhydrous and water-soluble forms (hydrogen fluoride solution in water). This product is classified in the category of weak acids and is also used in the production of aluminum and chlorofluorocarbons, glass engraving, and chemical industries. HF is in the category of Acids of Shanghai Chemex group Ltd.
Physical and Chemical Properties:
Hydrofluoric acid, also known as Fluorhydric acid, is soluble in water and many organic solvents, but this rate of dissolution in compounds such as ether is much lower than in other compounds. This compound, like other acids, has a very pungent odor and is a white gas that, when combined with water, will have a weaker acidity; In the table below some of the chemical and physical properties of HF are mentioned:
| Chemical formula | HF |
| Molecular Weight (g/mol) | 20.0064 |
| Appearance | Colorless liquid |
| odor | Strong, irritating odor |
| PH | In the water a weak acid |
| Density (g/cm3 at 25 °C) | 1.002 |
| Melting point (° C) | -83.57 |
| Boiling point (° C) | 20 |
| Solubility in water | completely miscible |
| Vapor pressure (mm Hg at 25 °C) | 917 |
| Flash Point | Not Flammable |
| Synonyms | Hydrogen fluoride, Fluorhydric acid, Fluorane, Hydrofluoride, Hydrogen-fluoride, Rubigine, Fluohydric Acid |
| Chemical Structure Depiction | 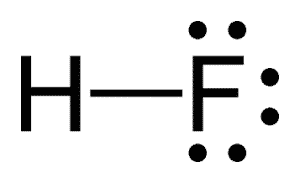 |
HF Production Process:
The First Method:
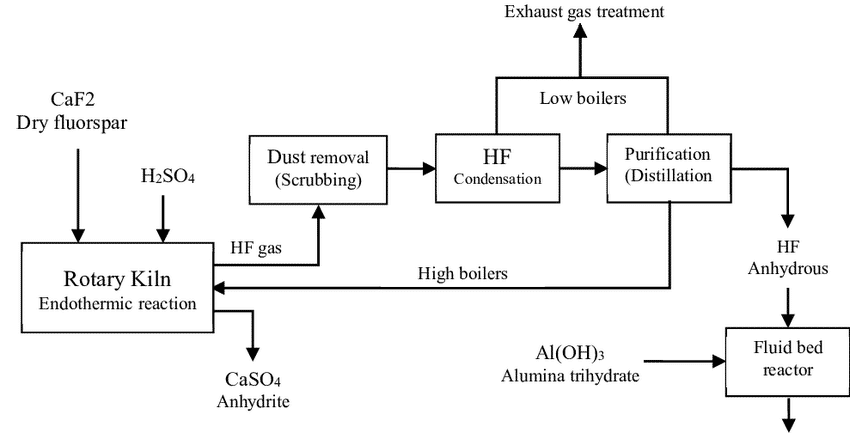
This strong acid can be produced by heating calcium fluoride with liquid sulfuric acid, and hydrogen fluoride gas and calcium sulfate are by-products of this reaction. This endothermic reaction usually takes place in a rotating tube furnace. In order to remove the produced gases (HF gas) and improve the production of HF acid, the whole process is done under a vacuum. Heating a rotary tube furnace with an internal combustion chamber is a common method used today to produce hydrofluoric acid. The use of 3 to 15 combustion chambers will improve energy control and conversion percentage.
The Process Generally Involves the Following Steps:
- Drying calcium fluoride
- The reaction of calcium fluoride and sulfuric acid
- Purification of hydrofluoric acid (removal of additives such as excess HF gas)
- In order to obtain HF acid with very high purity (98%), the pure liquid is distilled.
CaF2 + H2SO4 → 2 HF + CaSO4
The Second Method:
HF is obtained by dissolving hydrogen fluoride gas in water by distilling calcium fluoride with sulfuric acid.
Hydrofluoric Acid Uses:
The applications of the above product are generally classified into two categories: industrial and non-industrial uses. This product is used in the production of refrigerants, herbicides, gasoline, kitchen stainless steel products, aluminum, plastic, and incandescent lamps. About 60% of the consumption of the above product is in the production of refrigerants used in refrigerators, freezers, and air conditioning systems. Other uses for this compound include:
Industrial uses:
This acid is widely used in industry due to its redox properties and is also used as a solvent in industrial applications.
Cleansing Agent:
This corrosive substance can be used in many cleaners. In the case of metals, this acid can be used to remove oxides and other contaminants from the surface of carbon steel.
Production of Fluorides:
Hydrofluoric acid is used to produce high volume non-mineral fluorides such as cryolite, aluminum fluoride, etc.
Water Treatment:
In the water treatment sector, hydrofluoric acid is used as a disinfectant.

Carving:
Due to the high ability of HF acid to dissolve silica compounds, it is also used in carvings on stone, glass, and metal.
Oil Refining:
In the oil refining process, this acid is used as an acid catalyst.
Non-industrial uses:
Source of Fluoride:
In laboratory cases and reactions that require fluoride, fluoride acid is known as the source of fluoride.
Cleaners:
This acid can be used in cleaning and car care products and home furniture.
Hydrofluoric Acid for Sale:
Order registration in the shortest time with the guarantee of providing the product according to the analysis and with the best quality only in Shanghai Chemex.
Safety Information:
Although this product is a weak acid, it is very corrosive. For this reason, it is important to pay attention to the following points when working with this material:
This substance is so toxic and dangerous that if it comes in contact with the skin of the hand, it can cause severe burns and very deep wounds. When it touches the skin of the hand, it may not change immediately, but it will essentially destroy the tissues under the skin, and after a few hours, burn marks will appear on the hand over time. If the vapors and gases emitted by this acid come into contact with the eyes or are inhaled through the air, there may be irreversible risks. Therefore, to work with it, be sure to use filtered glasses, gloves, and a mask.
Handling and Storage:
Due to its high reactivity with glass and moderate reactivity with many metals, hydrofluoric acid is usually stored in plastic containers in a cool, well-ventilated environment.







LILI –
What household products contain hydrofluoric acid?
china chemicals –
Hydrofluoric acid is used in a variety of over-the-counter products such as toilet bowl cleaners, concrete cleaners, and metal polishes.