Description
Hydrochloric acid (HCl) is an inorganic acid that contains hydrogen and chloride ions. There is hydrochloric acid in the stomach; however, it can be produced in the laboratories, and synthesized HCl is used in a wide range of industries. HCl has a highly corrosive powder, so if you want to work with this substance, you should be aware of the safety information.
 How to buy hydrochloric acid?
How to buy hydrochloric acid?
If you would like to know any further information about the hydrochloric acid price or buying this item, our team at Shanghai Chemex will be glad to help you with that. You can contact us through our website or by calling our customer service hotline. We are committed to providing you with the best service possible.
Physical and Chemical Properties of HCL:
HCL appears as colorless liquid. This acidic substance has a very high corrosive power, and is a very practical option for scaling. In the table below, some of the chemical and physical properties of this product are mentioned:
| Chemical formula | HCl |
| Molecular Weight(g/mol) | 36.46 |
| Appearance | liquid |
| odor | Pungent, irritating odor |
| Density(g/cm3 ) | 1.639 |
| Melting point(° C) | -114.22 |
| Boiling Point(° C) | -85.05 |
| Solubility | Soluble in water, ethanol, methanol |
| color | Colorless |
| form | Liquid |
| Vapor Pressure(mm Hg) | 413.6 |
| hydrochloric acid structure | 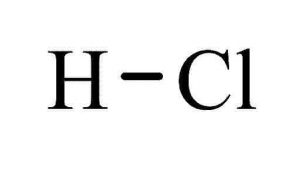 |
Hydrochloric acid’s pH is somewhere between 1.5 and 3.5.
How to prepare hydrochloric acid?
Hydrochloric acid is prepared by dissolving hydrogen chloride in water. There are many other ways to make hydrochloric acid. Industries heat NaCl with concentrated H₂SO₄. If the gas passes through a concentration of sulfuric acid, it will dry. Here are the reactions:
NaCl+ H₂SO₄ → NaHSO4+ HCl
NaHSO4 + NaCl → Na2SO4+ HCl
“Most of the hydrochloric acid (hydrogen chloride) produced in the United States is a by-product of the chlorination reaction. After leaving the chlorination process, the gas stream containing hydrogen chloride proceeds to the absorption column, where concentrated liquid hydrochloric acid is produced by absorption of hydrogen chloride vapors into a weak solution of hydrochloric acid. The hydrogen chloride-free chlorination gases are removed for further processing.”
Difference between hydrochloric acid and muriatic acid?
Both of these chemicals have the same formula. However, they are not the same. Hydrochloric acid is pure. It only contains hydrogen and chloride ions and appears as a clear solution. However, muriatic acid has impurities such as H₂SO₄, and in the presence of impurities, it appears as a yellow solution.
In addition, unlike hydrochloric acid, muriatic acid is not used in the laboratory of analytical chemistry.
Hydrochloric acid uses:
HCl has lots of applications in various industries, which are as follows:
- Chemical laboratories
It is used as a chemical reagent in the production of organic compounds such as vinyl chloride, plastic PVC, and dichloromethane. HCl is also used in inorganic compound synthesis, such as PAC, which has a strong role in water treatment.
- Food industry
In the food industry HCl is used as an additive to control the pH level of the food products.

- Removing stains
Due to its high degree of corrosion, HCl is frequently used as a chemical to remove stains or rust, particularly from metals like iron, copper, and others.
Does Hydrochloric acid Raise or Lower PH?
Hydrochloric acid can be used to regulate the acidity of solutions. In industries where the purity of products is very important (food, pharmaceuticals, drinking water), high-quality HCl is used to control the pH of water flows in processes. Normal quality HCl is sufficient to neutralize effluent flow and control the pH of the swimming pool.
Hydrochloric Acid Hazards:
- Vapors and droplets of this substance can cause severe irritation, burns, and blindness.
- It can cause severe skin irritation, redness, blisters and pain, burns, and skin injuries.
- It can cause corrosive sores in the mouth, throat, esophagus, and abdomen. Symptoms include difficulty swallowing, thirst, vomiting and nausea, diarrhea, severe injuries, coma, and death.
- The vapors of this substance can cause severe irritation of the nose, sore throat, and cough.


First-aid measures:
- Hydrochloric acid on the skin can cause burning. You should wash the exposed area of skin with water for 15 minutes.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
Keep the container tightly closed in a cool, dry, ventilated area away from incompatible materials.
Frequently asked questions
Is hydrochloric acid harmful to humans?
If this acid contacts the skin and eyes, it could be dangerous. It is able to cause severe burning and blindness, so you must use appropriate clothing and glasses while using this substance.
Is HCl a hydrochloric acid?
Yes, because hydrochloric acid contains hydrogen and chloride ions. It appears as a colorless solution, which is briefly known as HCL.
How powerful is hydrochloric acid?
It is considered a strong acid with a pH of 1.6. HCl is highly corrosive, and it can cause burning if it comes into contact with the skin.
Where is hydrochloric acid found?
It can be found naturally in the gases of volcanoes. More than that, the stomachs of mammals have HCl too.
Is hydrochloric acid safe for the skin?
If the low concentration of HCl contacts the skin, it can cause inflammation, and in higher concentrations, HCl is able to cause burning.






karen –
Do vinegar and salt make hydrochloric acid?
china chemicals –
When vinegar is mixed with salt, the acetic acid in the vinegar reacts with the sodium chloride or salt to produce sodium acetate and hydrochloric acid.