Description
Potassium carbonate is an inorganic compound with the chemical formula K2CO3, also known by other names such as potash carbonate, salt of tartar, and pearl ash. This chemical compound belongs to the family of salts, which has strong alkaline properties and is used in various industries such as food, detergent and hygiene, textile, glass, and chemical fertilizers. Potassium carbonate is in the category of food additives of Shanghai Chemex group Ltd.

Physical and Chemical Properties:
Potassium carbonate is a white, salt flavored white powder that is soluble in water but not soluble in ethanol, acetone, ether, and general organic solvents. It is a moisture absorber and its dissolution in water is a thermal process. Once dissolved in water, it ionizes and decomposes into its constituent ions. This compound is non-flammable, non-explosive, and nontoxic; In the following table, you can see some of the properties and characteristics of this product:
| Chemical formula | K2CO3 |
| Molecular Weight(g/mol) | 138.205 |
| Appearance | solid |
| Odor | Odorless |
| Taste | Alkaline taste |
| Density (g/cm3) | 2.43 |
| Melting point (° C) | 891 (1,636 °F; 1,164 K) |
| PH | 11.5 -11.6 |
| Color | White |
| Form | powder |
|
Chemical Structure Depiction |
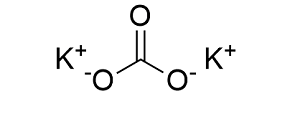 |
Potassium Carbonate Production Process:
Today, potassium carbonate is produced on a commercial scale using electrolysis of potassium chloride in the presence of carbon dioxide. On an industrial scale, the most efficient production method is the use of a fluidized bed reactor. As you can see below, water is a by-product of this reaction. Using a fluidized bed reactor, we can produce the product without any additional water. The process begins when potassium chloride produces potassium hydroxide, chlorine gas, and hydrogen gas during an electrolysis reaction. This reaction has very valuable by-products. Hydrogen can be used separately as a clean fuel. Chlorine gas also has very diverse and valuable applications. Liquid potassium hydroxide and carbon dioxide are the only raw materials needed to produce potassium carbonate. Dry potassium carbonate can be easily dissolved in water. Generally, this product is sold as a liquid in a concentration of 47%. Because the transfer of solution with this concentration reduces the problems caused by its phase change due to climate change. The chemical equation for this process:
2 KCl + 2 H2O → 2 KOH + H2 + Cl2
2 KOH + CO2 → K2CO3 + H2O
Potassium Carbonate Uses:
- One of the most important applications of this compound is in the field of agriculture and the production of agricultural fertilizers. Potassium carbonate is one of the three main nutrients needed by plants. Plant roots are strengthened by this inorganic compound.
- This compound is used in the chemical industry as a raw material for the manufacture of chemical compounds.
- This inorganic compound is a dryer and is used to dry ketones and alcohols before distillation.
- This compound is used as a cooking ingredient in some countries with certain conditions and amounts.
- The most common use of potassium carbonate is in soap making, which is often soft and liquid.
- It is also used in the textile industry. Potash is widely used for washing and dyeing leather products.
- Potassium carbonate is used to make special glass.
- Other applications include making glazes for pottery and producing pigments, and inks.
Is Potassium Carbonate Safe in Food?
This chemical compound is known as a safe additive in food and drug organizations in Europe and the United States, and its use as an additive in the food industry has not been reported to have any adverse or destructive effects. In general, compounds containing potassium are useful for the human body and increase bone density, cardiovascular health, etc. Potassium carbonate is also used as a regulator of food acidity in the production of beverages, chocolate, confectionery, custard powder, and Exercise supplements are used.
Does Potassium Carbonate Soften Water?
Magnesium and calcium are two causes of water hardness, and the use of potassium carbonate prevents the accumulation of calcium and magnesium in water and prevents the deposition of these salts in the piping system. If this substance is used in high doses, it can cause skin irritations and allergies, but in moderate and low concentrations, it will not pose a threat to human health.
Buy Potassium Carbonate:
Shanghai Chemex is currently one of the suppliers of chemical products in China, which sells potassium carbonate in food and industrial grades with the best price and quality. Contact our experts to buy the product and place an order.
Safety Information:
Potash carbonate, in dry or soluble form, irritates the eyes, skin, and respiratory system and can cause inflammation of the skin, eyes, throat, and stomach.
First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and storage:
Store in a dry and cool place, away from strong acids with proper ventilation in a tightly closed container.






George –
What is the common name for potassium carbonate?
china chemicals –
Potassium carbonate is also known as pearl ash.