Description
Sodium nitrite is a highly volatile mineral compound and is available in both liquid and powder forms. Used as a precursor to organic compounds, it is used in industries such as dyes, pesticides, pharmaceuticals, and the food industry. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.
Physical and Chemical Properties:
Sodium nitrite is an organic chemical with the formula NaNO2, which has the appearance of a pale yellow crystalline powder that is soluble in water and absorbs moisture; In the table below some of the chemical and physical properties of this product are mentioned:
|
Chemical formula |
NaNO2 |
| Molar mass (g/mol) | 68.9953 |
| Appearance | solid |
| Taste | Slightly salty taste |
| pH | 7-9 |
| Density (g/cm3) | 2.168 |
| Melting Point (° C) | 271 |
| Boiling point (° C) | 115 |
| Solubility in water (g/100 g water at 25 °C) | 84.8 |
| Solubility | moderately soluble in methanol, ethanol, and ammonia;
sparingly soluble in diethyl ether |
| Color | Slightly yellowish or white |
| Form | crystals, pellets, sticks, or powder |
| Chemical Structure Depiction | 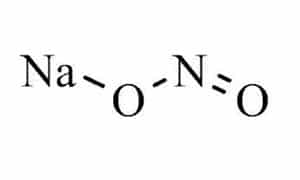 |
The Production Process of Sodium Nitrite:
Industrial production of sodium nitrite is done by one of two processes: the reaction of reducing nitrate salts or the oxidation of nitrogen oxide with lower degrees.
- A common method for producing sodium nitrite is to use a general reaction of nitrous oxide in an alkaline aqueous solution with the addition of a catalyst. The reaction conditions depend on the degree of oxidation of nitrogen oxide as well as the oxidizing agent.
- Sodium nitrite is also produced by reducing nitrate salts. This reduction is accomplished by exposing nitrate salts to heat, light, ionizing radiation, metals, hydrogen, and electrolytes. In this method, molten sodium nitrate is used as the reduced salt, and lead is used as the oxidizing agent. But today, in a more modern way, iron scrap is used as an oxidizing agent to perform the nitrate reduction reaction.
The Difference Between Sodium Nitrite and Sodium Nitrate:
The main difference between nitrate and nitrite is the bonding of their oxygen atoms. Nitrate contains three oxygen atoms attached to one nitrogen atom, while nitrite contains two oxygen atoms attached to one nitrogen atom. Nitrate and nitrite are mineral anions that are composed of nitrogen and oxygen atoms and have a negative electric charge. In fact, there are general differences between nitrate and nitrate.
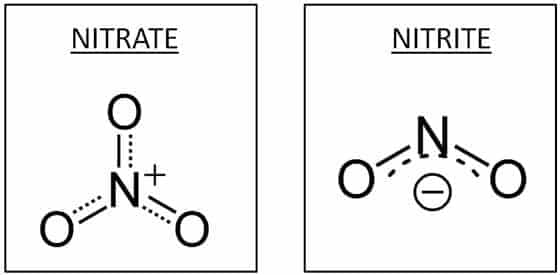
Sodium Nitrite Uses:
This combination is available in both food and industrial grades in the market. In the following, we will get more familiar with the applications of this combination in food and other industries:
Food:
Sodium nitrite is primarily used to prevent the growth of microorganisms, especially Clostridium botulinum, to preserve color and flavor in meat products. like Tripotassium Phosphate
Pharmaceutical:
Sodium nitrite is an essential ingredient for the World Health Organization. One of the reasons is that it is effective in relieving cyanide poisoning. Also, the combination of this substance with sodium thiosulfate can be used in the production of some drugs.
Industry:
The application of sodium nitrite in the industry is mostly for the industrial production of nitrogenous organic compounds.
Is Sodium Nitrite found in Meat?
In the distant past, salt was used to preserve meat. Salt over time caused the meat to turn black, so they looked for a suitable alternative to salt. As the industry progressed, they used sodium nitrite along with salt. By using sodium nitrite and salt simultaneously, the shelf life of the meat is increased and its color turns pink over time. Also, the taste of meat improves; Therefore, it is used as a preservative in food. One of the most common uses of this substance is in sausages, bacon, hot dogs, and ham. The first use of this substance in food dates back to the early twentieth century. Unauthorized use of add-ons was common at the time. Using this substance in meat and gathering information, they concluded that the use of this substance is safe and helps the taste and longevity of meat. Over time, this was proven and the use of this substance increased dramatically.
What is Sodium Nitrite used to Manufacture?
Sodium nitrite is a reactant for the conversion of amine compounds to diazo, which is widely used as a precursor for the production of dyes and pigments. Other uses of this product include the production of insecticides and herbicides, as a corrosion inhibitor, in the textile industry as a bleaching agent, in the rubber industry as a polymeric inhibitor, and in applications including water purification, antifreeze, industrial cleaners, and Lubricants noted. Due to its anti-corrosion properties, it is also used as a coolant in thermal energy storage for air conditioning systems and industrial refrigeration.
Buy Sodium Nitrite:
Shanghai Chemex is currently one of the leading suppliers of chemical products in China, offering a wide range of chemicals, including sodium nitrite. Contact our experts for more information on product prices and how to place an order.
Side Effects:
Despite all the benefits listed for using this substance, its use can also have risks. An article published in 2015 showed that long-term consumption of 0.1 mg of this preservative in meat products can increase the risk of gastric cancer by 7%. In 2017, a study on laboratory animals found that exposure to high doses of sodium nitrite reduced oxygen levels in the body and caused brain damage, anxiety, and depression.
Packing and Storage:
Store in a tightly-closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.








Reviews
There are no reviews yet.