Description
Propylene carbonate, also called PC, is an organic compound with the chemical formula CH3C2H4O2CO. PC is a carbonate ester derived from propylene glycol that absorbs moisture. This compound is used as an apprentice and polar solvent. Propylene carbonate is one of the important applications of this material in the pharmaceutical and cosmetic industries as a solvent. like salicylic acid

Physical and chemical properties:
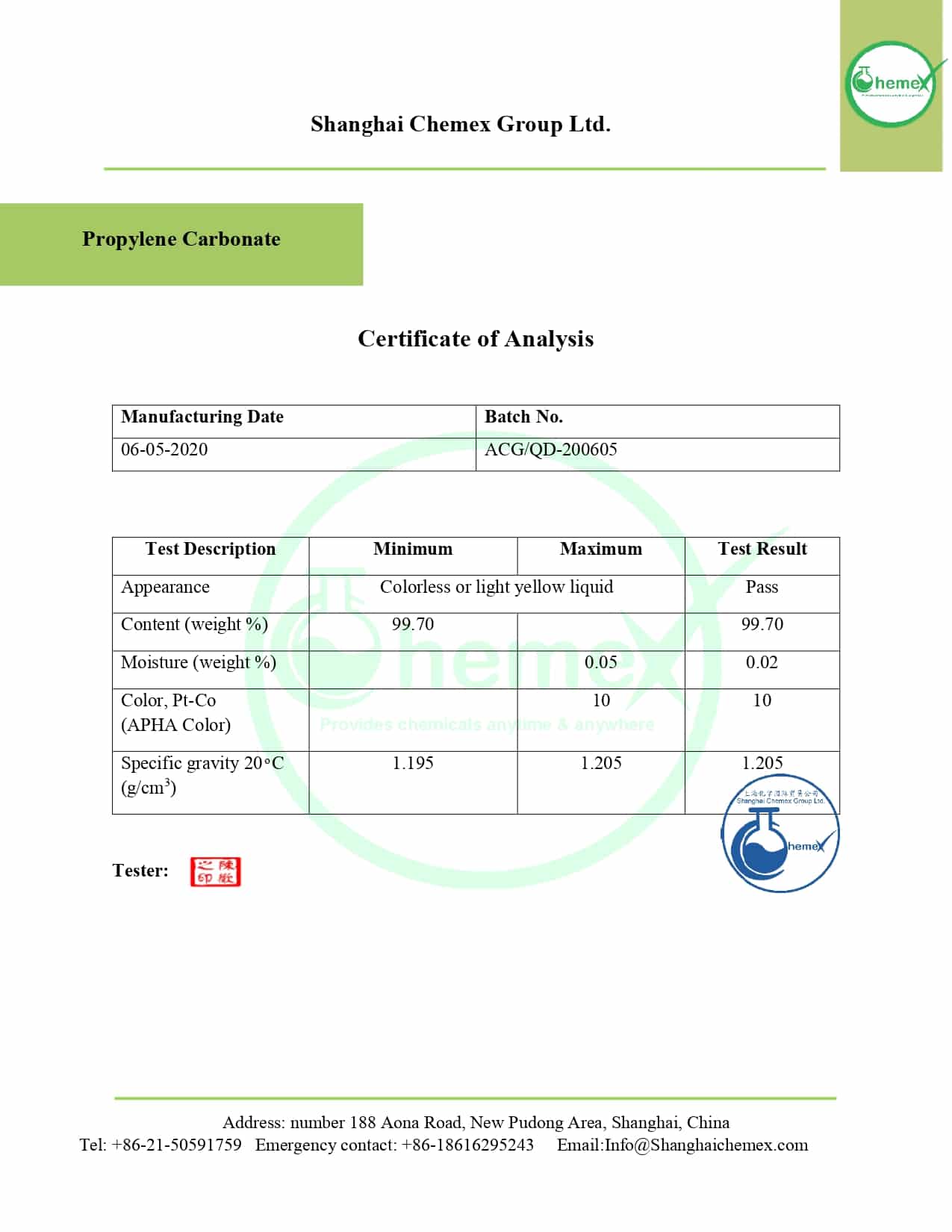
Propylene carbonate (PC) is a colorless, odorless liquid that can be mixed with water, acetone, benzene, and ether. It is a chiral substance but is often used as a racemic mixture. Propylene carbonate is a dipole molecule with more polarity than acetone and ethyl acetate.
| Chemical formula | C4H6O3 |
| Molar mass | 102.089 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.205 g/cm3 |
| odor | Odorless |
| Melting Point | −48.8 °C (−55.8 °F; 224.3 K) |
| Boiling point | 242 °C (468 °F; 515 K) |
| Solubility in water | Very soluble (240 g/L at 20°C) |
| Solubility | Very soluble in ethanol, ether, acetone, benzene
Miscible with chloroform and ethyl acetate; moderately soluble in carbon tetrachloride |
| vapor pressure | 0.045 mm Hg at 25 °C |
| Flashpoint | 116 °C (241 °F) |
| Color | Clear, colorless |
| Form | liquid |
| Other names | cyclic propylene carbonate
Carbonic acid, propylene ester Cyclic 1,2-propylene carbonate Propylene glycol cyclic carbonate |
| Chemical Structure Depiction | 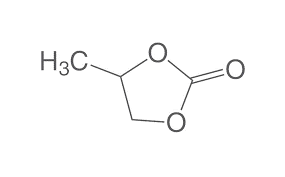 |
Propylene carbonate is a green solvent:
Among organic solvents, organic carbonates are considered green solvents. These esters are stable and can be used in both research and industrial applications. PC, as a member of the carbonate family, has properties that convert it into a green solvent.
Propylene carbonate production process:
There are several methods for producing this chemical compound, but the most widely used is to combine propylene glycol with carbon dioxide in the presence of acetonitrile.
CH3CHCH2O + CO2 → CH3C2H3O2CO
This organic compound is produced by the reaction between urea and 1.2- propylene oxide in the presence of a catalyst on acetate.
Other methods for synthesizing this compound include the ring-forming reaction of propylene oxide and carbon dioxide in the presence of various catalysts.
Propylene carbonate Uses:
- It is used as a solvent in the formulation of cosmetics such as lipstick, eye shadow, mascara, and…
- Used as an operatic and polar solvent with dipole moment beyond acetone and ethyl acetate.
- Propylene carbonate can be used as the main material for the composition of dimethyl carbonate and is widely used in the removal of CO2 and H2S from natural gas.
- Used to produce adhesives.
- It is used in the production of plastic materials to increase the elasticity and reduce the viscosity of materials.
- Used in the textile industry, printing, and dyeing.
- Approved as an indirect food additive by the Food and Drug Administration.
propylene carbonate in the cosmetics industry:
Propylene carbonate is not corrosive, meaning it does not spoil, so it can be used as a solvent in lipsticks, skin cleansers, eye shadows, and mascaras. It is suitable for this industry as it is colorless and odorless and is an incorruptible liquid.

Propylene carbonate as a cleaning agent:
- Board and electronic components cleaner.
- Washing polyurethane
- Carburetor cleaners
- Cleaning polymer/resin
- As a cleaner for painted surfaces.
- Cleaning of unsaturated polyesters.

Safety information on propylene carbonate:
Propylene carbonate in the use of cosmetics does not irritate the skin or make the skin sensitive, while in the use of a solution of this substance, skin irritation is observed. Swallowing, inhaling, and making eye contact can be very dangerous.

First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person into the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and storage:
Store in a cool, dry, well-ventilated area away from incompatible substances. Keep containers tightly closed.






Reviews
There are no reviews yet.