Description
Iron (III) chloride, also known as Ferric chloride, is one of the inorganic compounds used in various industries, which is produced by the reaction of iron and chlorine in special conditions. The chemical formula of this inorganic compound is FeCl3 and it is used in powder, liquid, and solid forms in various industries. This compound is one of the strongest coagulants that has acidic properties and has a very high corrosive power. These compounds are used in various industries such as metal extraction, pharmaceuticals, dyeing, paper making, arsenic removal, water treatment, and wastewater treatment. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
The compound is hydrolyzed when dissolved in water to form a highly corrosive brown acidic solution with a weak hydrochloric acid odor that is used as a coagulant in wastewater treatment and drinking water production. Anhydrous iron chloride (III) is a strong Lewis acid that is used as a catalyst in organic synthesis. This substance is available in powder, liquid, and solid form and the most common use of ferric chloride is in solution; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | FeCl3 |
| Molecular Weight(g/mol) | 162.204 (anhydrous),
270.295 (hexahydrate) |
| Appearance | Liquid |
| odor | weak hydrochloric acid odor |
| Density (g/cm3 at 25 °C) | 2.90 |
| Melting point (° C) | 307.6 (anhydrous),
37 (hexahydrate) |
| Boiling point (° C) | 316 (anhydrous, decomposes),
280 (hexahydrate, decomposes) |
| Water Solubility (g/100 cc)
|
In cold water at 0 °C: 74.4,
in hot water at 100 °C: 535.7 |
| Solubility | Slightly soluble in carbon disulfide, practically insoluble in ethyl acetate |
| Vapor pressure (mm Hg at 381 °F) | 1 |
| Other names | Ferric chloride, Molysite, Flores martis, Iron(III) chloride, Iron trichloride |
| Color | colorless to light brown |
| Form | aqueous solution |
| Chemical Structure Depiction | 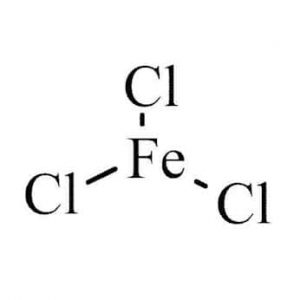 |
Types of Ferric Chloride:
Ferric chloride is divided into different types based on their appearance, which are:
Powder:
This compound is water-free and has a very high moisture absorption power. The color of this compound is brown in powder form and turns orange if it absorbs moisture.
Liquid:
The color of this liquid is garlic brown. This compound has a highly acidic odor and is non-flammable. Liquid iron chloride in very high concentrations contains six molecules of water (FeCl3.6H2O) which is called a 6-aqueous crystal and is yellow.
The Production Process of Ferric Chloride:
Ferric chloride is produced industrially by a process called direct chlorination, the reaction of dry chlorine with scrap iron at a temperature of 500 to 700 ° C. Under the influence of high temperature, chlorine and iron react together and iron is gradually oxidized to iron (II) chloride. This compound is converted to Iron (III) chloride, or Ferric chloride, in response to the abundant chlorine in the environment.
This compound can also be prepared from the reaction of hydrochloric acid with iron. In this reaction, iron reacts with hydrochloric acid and then reacts with chlorine. In these reactions, powdered, crystalline, and liquid iron chloride can be obtained by changing the temperature and environmental conditions.
Fe2O3 + 6HCl → 2FeCl3 + 3H2O
Ferric Chloride Uses:
- In sewage treatment and drinking water treatment;
- As a disinfectant;
- Preparation of plant fertilizers;
- Electronics industry;
- Extraction of metals;
- In Glass and ceramic industries;
- Pharmaceutical industry (raw material for the production of drugs in the treatment of anemia);

- Paint industries;
- Paper industry;
- Removal of phosphate and arsenic;
- In the laboratory as Lewis acid;
- As a catalyst in reactions such as Friedel-craft aromatics and chlorination of aromatic compounds;
- As a raw material in the production of pigments, dyes, and cosmetics.
Safety Information:
Ferric chloride is harmful, highly corrosive, and acidic, so be sure to use caution when working with this substance. Although poisoning is rare in humans, the use of ferric chloride can lead to serious mortality.


First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
The material should be stored in a tightly-closed container in a cool, dry, well-ventilated area away from incompatible substances.






Reviews
There are no reviews yet.