Description
Benzoyl Peroxide is an organic peroxide with a chemical structure (ChH5 C (= O) O−) 2. This substance is also known by the acronym BPO or 2 (BZO). Benzoyl peroxide is a well-known substance in the food and pharmaceutical industries. It is used in the food industry as a whitening agent and in medicine as a raw material for the production of skin care products in the form of gels or other forms to eliminate acne. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Benzoyl Peroxide is a white granular crystalline solid with a weak odor of benzaldehyde that has little solubility in water but is soluble in other solvents such as acetone, ethanol, and many other organic solvents; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | C14H10O4 |
| Molar mass (g/mol) | 242.23 |
| Appearance | solid |
| Density (g/cm3) | 1.334 |
| Odor | benzaldehyde-like odor |
| taste | Tasteless |
| Melting Point (°C) | 106-108 |
| Solubility | Sparingly soluble in alcohol; soluble in water, benzene, chloroform, and ether |
| color | White |
| form | granular crystalline |
| Chemical Structure Depiction | 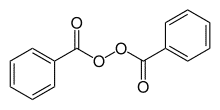 |
Types of Benzoyl Peroxide in the Market:
- Aqueous powder;
- powder without water;
- Suspended benzoyl peroxide paste
Production of Benzoyl Peroxide:
This substance is the first synthetic organic peroxide that was synthesized according to the following reaction.
- Production of benzoyl peroxide from benzoyl chloride and barium peroxide:
- 2 C6H5C(O)Cl + BaO2 → (C6H5CO)2O2 + BaCl2
- Production of benzoyl peroxide through the reaction between benzoyl chloride with oxygenated water (hydrogen peroxide) and sodium hydroxide:
- 2 C6H5COCl + H2O2 + 2 NaOH → (C6H5CO)2O2 + 2 NaCl + 2 H2O
- The bond between the two oxygen atoms is weak, so benzoyl peroxide is easily hemolyzed (symmetrical fission) and forms free radicals:
- (C6H5CO)2O2 → 2 C6H5CO2
Benzoyl Peroxide Uses:
This compound is used as a bleach in some foods, an oxidizing agent, a polymerization initiator in the production of plastics, a baking agent in silicone rubber, part of the drugs used for skin disorders, and as a raw material in some industrial processes.
- This substance is used to treat and reduce the number of lesions caused by acne on the skin. The use of this substance does not cause antibiotic resistance.
- Benzoyl peroxide is one of the most important organic peroxides in terms of application and production scale. It is often used as a suitable oxidant in organic chemistry.
- Like most peroxides, it is a strong bleach. It is used to whiten flour, fats, oils, waxes, and cheeses and as a stain remover.
- It is also used as a radical initiator to induce chain-growth polymerization reactions.
How To Treat Acne with Benzoyl Peroxide
Benzoyl peroxide is used in medicine to treat acne. As a disinfectant, this substance reduces the number of microbes (bacteria) present on the surface of the skin, which is available as a gel or face wash with a purity of 5 and 10%; But there is no reason for the drug to be more effective if the concentration is increased. like salicylic acid. Some other forms of BPO, which also contain antibiotics and exfoliators, can only be obtained with a prescription.
Buy Benzoyl Peroxide:
For more information on purchasing and ordering this product, please contact our experts in Shanghai Chemex through the numbers on the site.
Safety Information:
Some people are very sensitive to benzoyl peroxide. May irritate the skin, eyes, and respiratory tract. May be harmful if swallowed or inhaled. Serious allergic reactions can occur, including rash, swelling of the mouth and tongue, difficulty breathing, and dizziness.



First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
Store in a tightly-closed container, in a cool, dry, and ventilated area away from heat and fire.






Reviews
There are no reviews yet.