Description
Sodium hydroxide is a highly potent chemical used in many industrial solvents and cleaners. Industrially, sodium hydroxide may be used in the production processes of products such as paper, plastics, soap, and rayon textiles. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.
Physical and Chemical Properties:
Sodium hydroxide, caustic soda, has the chemical formula NaOH. This is a white solid that has a boiling point of 1390 ° C. It is a water absorber and absorbs water from its surroundings. For this reason, it is necessary to take the necessary precautions in maintaining it. In contact with water, this substance is completely separated from its constituent ions and has alkaline properties. It dissolves well in ethanol, methanol, and water.
| Chemical formula | NaOH |
| Molecular Weight (g/mol) | 39.9971 |
| Appearance | solid |
| odor | odorless |
| Density (g/cm3) | 2.13 |
| Melting point (° C) | 323 (613 °F; 596 K) |
| Boiling point (° C) | 1,390 (2,530 °F; 1,661 K) |
| Solubility in water (g/L at 25 °C) | 1000 |
| Solubility | soluble in glycerol, ammonia, propylene
insoluble in ether |
| Vapor Pressure (mm Hg) | 0 |
| Viscosity (cP at 350 °C) | 4 |
| Color | Colorless to white |
| Form | flakes, beads, granular form |
| Chemical Structure Depiction | 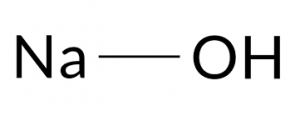 |
Production Process:
Sodium hydroxide can be obtained by reacting sodium carbonate with calcium hydroxide by the reaction.
Ca(OH)2(aq) + Na2CO3(s) → CaCO3 ↓ + 2 NaOH (aq)
The highest amount of this compound produced is obtained through the process of electrolysis of sodium chloride solutions:
2NaCl + 2H2O → 2NaOH + H2 + Cl2
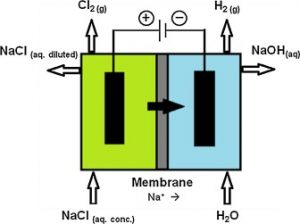
Caustic soda is also produced by the direct reaction of sodium and water, the by-products of which are gas hydrogen, and heat, which are often accompanied by a flame.
Sodium Hydroxide and Potassium Hydroxide:
Potassium hydroxide and sodium hydroxide are both highly corrosive and strong bases. Because these compounds are both strong bases, they react with water to release heat, so both are highly exothermic. Their chemical similarity also leads to their physical similarity that is similar to them. Also, the purchase and export of potassium hydroxide are as high as the purchase of sodium hydroxide in the world.
Sodium Hydroxide Uses:
Sodium hydroxide is used in the manufacture of many everyday products such as paper, aluminum, stove cleaners, and soaps and detergents, Some of its applications are:
- This compound is used to make a variety of soaps and detergents used in homes and commercial applications. Javelin (or Vitex) is produced by combining liquid chlorine and sodium hydroxide.
- It is used to help produce a variety of drugs and pharmaceutical products such as aspirin, cholesterol-lowering drugs, and blood clot-preventing drugs.
- In the energy sector, it is used in the production of fuel cells. Fuel cells such as batteries are used in the generation and storage of electricity for a wide range of applications, including transportation and wind turbines.
- Municipal water treatment plants use sodium hydroxide to control the acidity of water and help remove heavy metals from the water.
- In the paper recycling process, sodium hydroxide is used to separate the ink from the paper fibers.
- This valuable material is used to produce rayon, spandex, explosives, epoxy resins, paints, glass, and ceramics.
- It is also sometimes used in the fertilizer industry and the sale of chemical fertilizers.
Sodium Hydroxide in Paper and Wood production:
In many paper-making processes, wood is added to a solution containing sodium sulfide and sodium hydroxide. This helps to dissolve most of the junk and excess material in the wood and removes the relatively pure cellulose that forms the base of the paper. In the paper recycling process, sodium hydroxide is used to separate the ink from the paper fibers, which allows the paper fibers to be re-prepared for reuse, as well as to modify raw materials for wood products such as cabinets and furniture, and bleach and clean wood.
What is Caustic Soda used for in Water Treatment?
Another application of sodium hydroxide is in water treatment. Due to its alkaline properties, this chemical raises the pH of the water and reduces its corrosion. Therefore, it reduces the damage to the water pipes. This chemical also eliminates dangerous elements such as tin, copper, and other metals in the water, so it can greatly contribute to the health and cleanliness of the water.
Buy and Sell Sodium Hydroxide:
You can contact our experts in Shanghai Chemex to buy this product with high quality and reasonable price.
Safety Information:
Sodium hydroxide solution is a highly corrosive compound that can disrupt the structure of the body’s proteins and destroy them. It can also completely dissolve living tissues. One of the most serious dangers of this chemical to the body is the contact of this substance with the human eye, which if it comes in contact with the human eye, can cause permanent blindness in humans. Solid sodium hydroxide can also be highly corrosive and harmful to human health if exposed to water or water vapor. Therefore, when working with this compound, you should fully observe the safety measures and use a hat, gloves, special clothes, glasses, and other safety items completely to avoid any harm to the health of the person.
Packing and Storage:
Store this material in a cool, dry place. Barrels containing this compound can also be dangerous after being emptied. Due to the high absorption of carbon dioxide in the air by this compound, it is stored in air-resistant containers.




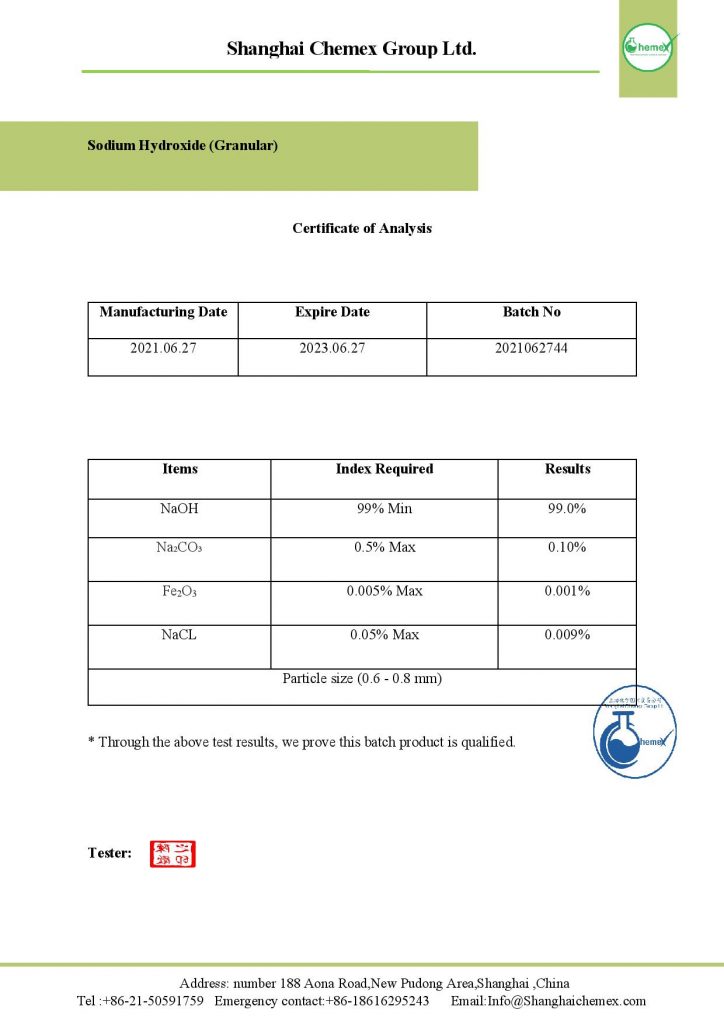




Reviews
There are no reviews yet.