Organic chemistry is a subset of the science of chemistry, which is the chemistry of carbon compounds. Until about 1850, many chemists believed that the source of organic compounds must be living things, and therefore they could never be synthesized from inorganic; But today, organic matter can be synthesized through industrial and laboratory methods and with the help of inorganic. Materials derived from organic sources have one common feature, which is that they contain the element carbon. The two major sources of organic matter from which simple compounds can be obtained are oil and coal, both of which are decomposed by animals and plants. These sources are used to prepare larger and more complex compounds.
Organic chemistry is the study of the structure, properties, composition, reactions, and preparation of carbon-containing compounds. These substances include not only hydrocarbons but also other compounds such as nitrogen, halogen, phosphorus, silicon, and sulfur.
What is a Major Difference between Inorganic and Organic Compounds?
Organic substance is present throughout the earth’s ecosystems and is produced through a cycle of degradation processes by soil microbes, which are crucial for access to nutrients. After degradation and erosion, these compounds can enter the soil through the flow of water. Organic matter provides food for living organisms and acts as a buffer in an aqueous solution to maintain a neutral pH in the environment. Research has shown that organic compounds may be an effective buffer for neutralizing acid rain.
An inorganic substance is a chemical compound that does not have hydrogen carbon bonds. Some simple compounds that contain carbon are often considered inorganic. Examples include carbon monoxide, carbon dioxide, carbonates, carbides, cyanides, cyanates, and thiocyanates.
General Properties of Organic Compounds:
Organic compounds are the basis of life on Earth, and most chemicals into this category. The physical properties of these materials include a variety of quantitative and qualitative properties. Quantitative properties include melting point, boiling point, and refractive index, and qualitative properties include odor, concentration, solubility, and color.
Classification of Compounds in Organic Chemistry:
Functional groups: There are certain groups of molecules that change the properties of a molecule. They cause molecules to participate in specific chemical reactions. Aldehydes, for example, participate in redox reactions. These compounds have many effects on physical and chemical properties. Most have heteroatoms, including alcohol, esters, carboxylic acids, amines, aldehydes, ketones and etc. Due to their electronegative properties, they make the molecule more acidic or alkaline.
Aliphatic compounds: Aliphatic compounds are divided into three groups: alkanes, alkenes, and alkynes. Alkanes have a unique and saturated bond. In alkenes, the carbon bond is a double carbon, and in alkynes, it is a triple bond. They can also be found in cycles (saturated and unsaturated). The smallest ring of cycloalkanes is cyclopropane.
Aromatic compounds: In chemistry, aromatic properties are given to compounds that have a ring structure and are flat. These compounds are very stable and are usually aromatic. A familiar example is the aromatic compound benzene. A familiar example is the aromatic compound Benzene.
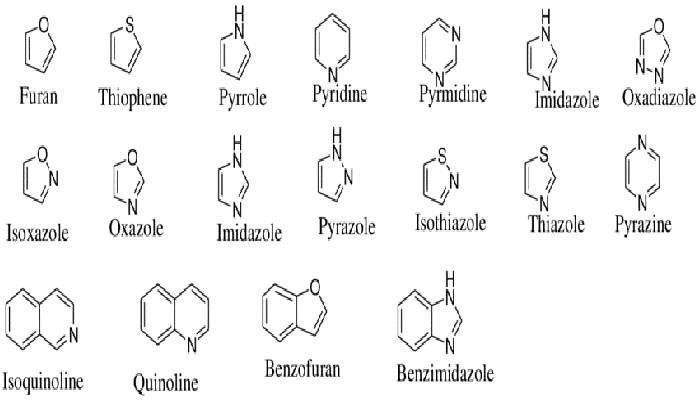
Heterocyclic compounds: They are cyclic organic compounds in which the ring-forming atoms are composed of two or more different atoms, such as pyridine, tetrahydrofuran, and isothiazolinone. The heteroatoms in these rings are usually oxygen, nitrogen, and sulfur. Heterocyclic compounds are widely used in the synthesis of organic compounds like solvents and reagents.
Polymers: One of the most important properties of carbon is that it can bind together in large numbers to form a long carbon chain. The process of bonding carbons together is called polymerization, and the chain or lattice formed is called a polymer. Each of the carbon units involved in the formation of carbon chains is also called a monomer. Two important groups of polymers are synthetic and biological. Among the synthetic polymers, we can mention the plastics that we use daily. Natural polymers that we have in our body such as proteins, amino acids and etc.
Biomolecules: Another group of organic compounds is biomolecules. These molecules, which are biopolymers, include peptides, DNA, RNA, polysaccharides, and cellulose. They can generally be grouped into amino acids, hydrocarbons, nucleic acids, and lipids.
Organic Chemistry Reactions:
Organic chemistry reactions are chemical reactions in which organic compounds are used as reactants. In many of these reactions, it is the functional groups that participate in the reaction. The general theory of these reactions involves a detailed analysis of properties such as electron affinity in atoms, bond strength, and spatial inhibition. In general, these compounds can perform a variety of nucleophilic substitution reactions, electrophilic, oxidation, reduction, etc.
What is the Role of Organic Chemistry in Modern life?
The clothes you wear every day are made of natural fibers such as cellulose and synthetic fibers such as nylon. The foods you eat all contain organic compounds such as protein and hydrocarbons. When you get sick and need medication to treat it, all of these medications use organic compounds. Many of these examples can be given to understand the importance of organic chemistry and its application in life.
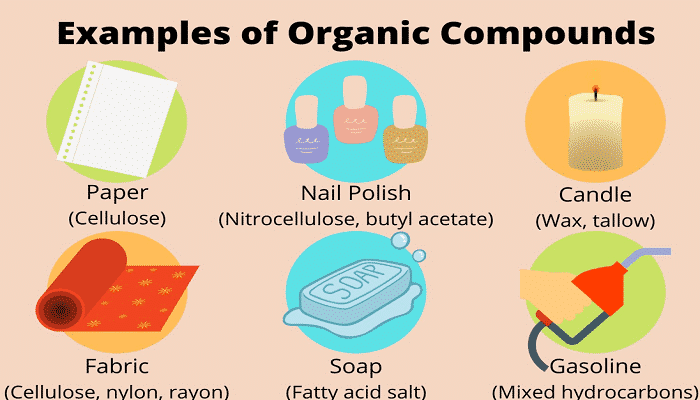
Today, this science is widely used in various industries. In this article, we tried to review the types of organic compounds and mentioned the importance of these compounds in everyday life.






2 Responses
What is the general importance of organic chemistry?
Organic compounds are important because all living organisms contain carbon. They are the basic components of many of the cycles that drive the earth. For example, the carbon cycle includes the exchange of carbon between plants and animals in photosynthesis and cellular respiration.