Citric acid is a weak acid found in citrus fruits and is referred to as an intermediate in the acid cycle, which is present in the metabolism of aerobic organisms. Citric acid’s formula is C₆H₈O₇. This compound has three groups of COOHs that can react with three open molecules, usually in the form of anhydrous or monohydrate.
Given the wide range of uses that have been introduced for citric acid, we need to have a brief overview of the most common neutralizing chemical reactions in which this organic acid participates. In many of these processes, this acid reacts with other raw materials, and in many other processes, it merely acts as a pH regulator.
Neutralization:
In chemistry, a neutralization reaction is a chemical reaction in which an acid and a base react quantitatively together to produce salt and water. Because in such reactions, the acidic and alkaline properties are neutralized, they are known as neutralization reactions. It is interesting to know that these types of reactions are also known as acid and base reactions. Note that no hydrogen ions, or hydroxides, will remain in the solution during the neutralization reaction.
Types of Neutralization Reactions:
There are three types of neutralization reactions, depending on whether the acid is strong or weak.
Strong Acid with a Strong Base:
Strong acids and strong bases are called acids and bases when they are completely separated in an aqueous solution.
It should be noted that when a strong acid is neutralized by a strong base, no excess proton ions or hydroxides remain in the solution. When a solution is neutral, it has no acidic or alkaline properties. The pH of this reaction is seven or close to seven.
Weak Acid with a Strong Base:
A weak acid is an acid that does not dissolve completely in water, such as citric acid. Therefore, the reaction will be in equilibrium. The pH of this reaction depends on the acid resolution (pKa). (PH>7)
Strong Acid with a Weak Base:
This reaction is similar to the reaction between a weak acid and a strong base. The weak base is called the base to partially dissociate in water. Here, the pH value at the endpoint of the titration depends on pKa and pKb. (PH<7)
Now that we are fully acquainted with the concept of this topic, we will discuss how neutralizing citric acid.
Neutralization of Citric Acid with Sodium Hydroxide:
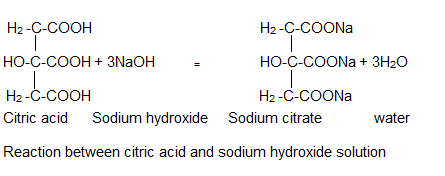
The chemical reaction between citric acid and sodium hydroxide is an acid-base neutralization reaction. In this reaction, an acid-base balance is established between the products and the raw materials. The reaction is as follows: Citric acid as acid and sodium hydroxide as an alkaline substance combine to form sodium citrate and water.
The reaction between citric acid and sodium hydroxide takes place in three stages; Because each citrate group must be replaced by a sodium atom. The compounds produced in 3 steps are monosodium citrate, disodium citrate, and trisodium citrate, respectively. Each compound has a defined acid dissociation constant, which increases from monosodium to trisodium, the acid dissociation constant, respectively.
Monosodium Citrate: C6H8O7+NaOH→ C6H7O7Na+H2O
Disodium Citrate: C6H7O7Na+NaOH → C6H6O7Na2+H2O
Trisodium Citrate: C6H6O7Na2+NaOH → C6H5O7Na3+H2O
- Here is citric acid + sodium hydroxide balanced equation:
Neutralization of Citric Acid with Sodium Bicarbonate:
If sodium hydroxide is not available, I can use a base such as sodium bicarbonate to neutralize citric acid.
Sodium bicarbonate, commonly called baking soda, is a chemical compound with the formula NaHCO3. The chemical composition of citric acid and sodium bicarbonate reacts together to produce another compound called sodium citrate, water, and carbon dioxide. Because this chemical reaction is associated with the production of carbon dioxide, the reaction conditions must be controlled in terms of temperature and pressure. Although no explosive reaction occurs, it is recommended that this reaction be performed under controlled conditions.
C6H8O7+3NaHCO3→ C6H5O7Na3+3H2O+3CO2
To perform the citric acid neutralization equation with sodium bicarbonate and to produce carbon dioxide, the temperature conditions during the reaction regulate the composition of the raw materials; It also affects the amount of carbon dioxide production as a final product; This is why we emphasize that temperature and pressure conditions must be adjusted during the reaction.
How to Neutralize Citric Acid in Food?
Citric acid is one of the most common food additives in the world and is used to enhance acidity, enhance flavor and preserve food ingredients.
The US Food and Drug Administration (FDA) has declared the use of this acid as a safe food additive. The citric acid consumed in the body is completely metabolized and does not cause any toxicity to the body. But this is somewhat different in relation to the industrial types of this acid. Because it has been observed that some people may be sensitive to foods that are high in citric acid. Therefore, to solve this issue, citric acid must be neutralized. For this purpose, we use sodium bicarbonate to neutralize it.
“Citric acid has also been used to adjust the pH of foods and cosmetics, as a sequestering agent to remove trace metals, and as a mordant to brighten colors.”
Frequently asked questions
How does NaOH neutralize citric acid?
The chemical reaction between citric acid and sodium hydroxide is an acid-base neutralization reaction. The reaction between citric acid and sodium hydroxide takes place in three stages; Because each citrate group must be replaced by a sodium atom.
Does water neutralize citric acid?
Water can affect the pH of the solution, but it is not enough to neutralize it.
How much NaOH does it take to neutralize citric acid?
How do you lower the pH of citric acid?
For neutralizing citric acid, you need to use a strong base such as sodium hydroxide (NaOH). In this reaction, an acid-base balance is established between the products and the raw materials.
How much sodium bicarbonate to neutralize citric acid?
The ratio should be 3:1. When these two materials react together, they produce another compound called sodium citrate, water, and carbon dioxide.







6 Responses
What is the best way to neutralize citric acid?
The best way to neutralize citric acid is to use a strong base such as sodium hydroxide(NaOH). But if NaOH is not available, sodium bicarbonate can also be used.
How much baking soda does it take to neutralize citric acid?
That is for every 1 gram of citric acid use 1.3 g of baking soda.
Does sugar neutralize citric acid?
No, sugar is not able to change the pH, however it can enhance the flavor.