In general, plating and also electroplating processes are those processes in which a coating of different materials is placed on metals.
To be more precise, electroplating processes are performed to increase the resistance of various metals against undesirable factors such as abrasion and corrosion, according to the reasonable price of materials used in the electroplating process. This process is very economical.
Electroplating near us:
The following figure some of the tools we use near us in our daily lives:

Electroplating chemicals:
Many chemicals are used as additives in this process, the most important of which are discussed below. The use of the following materials is very effective in the beauty and brightening of the final part.
- Bis Benzene Sulfonyl Imide (BBI)
- PROPARGYL ALCOHOL MONO ETHANOL ETHER (PME);
- PYRIDINIUM PROPYL SULFONATE (PPS);
- SODIUM ALLYL SULFONATE (SAS)
Shanghai Chemex Company is one of the most reputable suppliers of these products in the world.
electroplating process:
To perform the electroplating process of these materials, electric force is required,
In the electroplating process, we have two different parts, the anode, and the cathode, usually, the piece of metal that we want to cover is placed in the cathode part, and the metal that performs the plating process is placed in the anode part.
The most common metals used in this process are silver, chrome, tin, gold, and lead.
Both parts enter a solution by connecting an electric current to the anode, the electric force causes the electrons to lose electrons, and so-called oxides are made, these electrons dissolve in solution and move to the cathode part by creating an electric current towards these bonds and are regenerated in the cathode part.
the thin layer on the desired piece, the surface, and the thickness of the formed layer directly depend on the duration of the electric current.
Read more: Purification of Industrial Wastewater

In general, the electroplating process is done for different purposes, creating beauty and polish in the piece, protecting the piece against undesirable factors and phenomena such as corrosion, and creating some properties that are the most important goals of using this method in coating the coating surface of metals.
A noteworthy point in plating processes is that any metal that we want to create a plating process on it needs an electrolyte solution based on the same metal.
For example, we need a gold-based electrolyte solution for gold metal plating, and for copper metal plating, we need a copper salt electrolyte solution.
Before starting the electroplating process, it is necessary to make sure that the electrodes are clean so that the process is high quality is done and a product of high quality is finally provided.
The next point to consider in this process is that it takes a while for the plated atoms to form on the surface of the negative electrodes.
Exactly how long it takes depends on the power of the electric current you use and the electrolyte concentration.
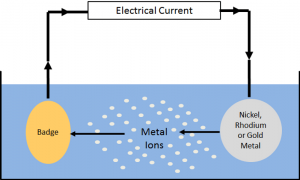
Increasing these parameters increases the speed of ions and electrons moving through the circuit and speeds up the plating process. As long as the ions and electrons are moving, it maintains the current and the plating process continues.






