EDTA, or ethylene diamine tetraacetic acid, is a chelating and complexing agent that binds to and traps many metal cations, which is why it is used in many research processes to separate or purify solutions. The most important uses of EDTA are the removal of disturbing ions in the pulp preparation process, cations in water and wastewater treatment plants, iron ions in water, magnesium and calcium cations in detergent production, and iron cations in agricultural production cited.
What is the Best Chelating Agent?
In selecting chelating, the type and amount of metal ions and anions present in the process must be considered. An important factor is the strength of the complex formed between the chelating agent and the metal ion. This factor determines whether the complex is formed in the presence of competing anions. The stability or equilibrium constant (K) displayed by log K is determined for many metals and chelating agents. Higher log K values indicate a stronger bond between the metal ion and the chelating agent, resulting in a greater likelihood of complex formation.
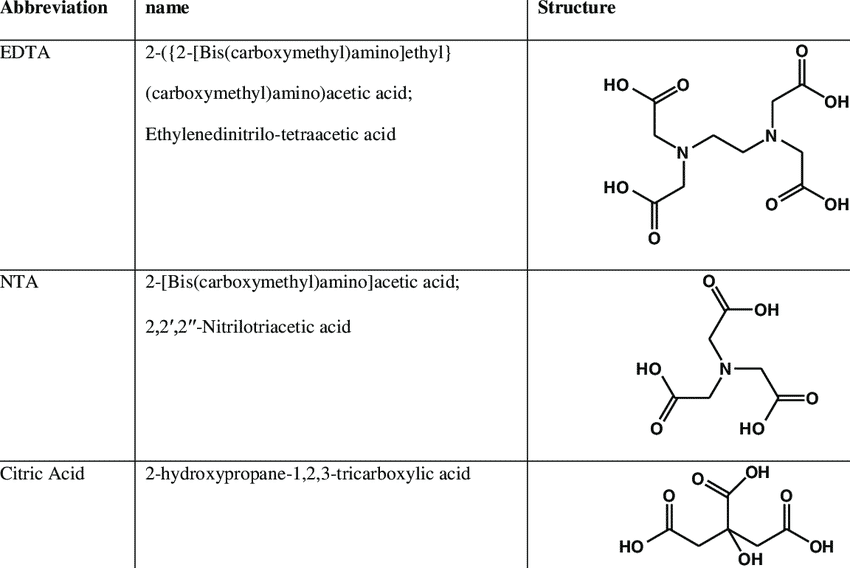
Among the most common chelating agents, the following can be mentioned, among which EDTA 2Na is the best type.
What is the Process of Chelation?
Metal ions have a great effect on the chemical processes and performance of many products. A wide range of problems related to metal ions can be solved by metal chelates and improve the efficiency of industrial systems. Chelating agents (often separating agents) can inhibit adverse catalytic reactions of the metal by forming a complex with metal ions and eventually inactivate the metal ion and prevent it from reacting with other components of the system. Among the various types of metal ion control agents that are used today, we can mention EDTA, which is one of the most effective metal ion controllers.
Benefits of chelated EDTA:
- Predictable performance
- High thermal stability
- Chemical stability
- PH stability
- Resistance to bacterial decomposition
What is EDTA Chelated used for?
Water Treatment:
Metal salts cause deposits in boilers, heat exchangers, and water circulation systems. EDTA, as a metal chelate, forms a stable complex with harmful metal ions and dissolves the formed sediments, and prevents the formation of new sediments.
Detergents:
Chelating agents, including the above product, are molecules that are able to form stable complexes with metal ions. These materials can be used in a wide range of industrial processes, but are used in detergent products to prevent soaps and detergents from reacting with mineral deposits in hard water and to improve soap quality.
Food Industry:
The reaction of heavy metals with organic and inorganic compounds in foods and beverages causes discoloration, texture change, and turbidity. To prevent these problems, EDTA as a chelating agent inactivates undesirable metals.
Oil Industry:
Chelating agents are widely used in petroleum applications. EDTA plays an important role in preventing the deposition of unwanted dissolved compounds including SrSO4, BaSO4, CaCO3, and iron deposits.
Paper Industry:
In the pulp and paper industry, EDTA also prevents the ability of metal ions, especially manganese, to disproportionately catalyze hydrogen peroxide used in chlorine-free bleach.
Textile Industry:
In the textile industry, EDTA prevents the effects of metal ions impurities on the color change of colored fabrics.







4 Responses
Is chelated EDTA also used in agriculture?
Metal chelates are a combination of metal ion bonds on an organic molecule (ligand). Metal ions are among the most important minerals for plants. These metals are used in small quantities by the plant and for this reason, they are called “micronutrients”. Deficiency of these elements leads to yellowing of leaves, slow growth, and an overall decline in product quality. Chelate compounds are more stable than non-chelate types. Therefore, metal chelates are widely used in agriculture and are provided to the plant in the form of micronutrient fertilizers to provide elements such as iron, manganese, zinc, and copper. The most common chelate fertilizers used in agriculture are EDTA, DTPA, and EDDHA.
Which metals does EDTA chelate?
Mg2+, Cu2+ and Fe2+ are the ions which can be chelated by EDTA. It can also chelate Mn2+, Ni2+ and Zn2+.