In general, chemical solvents are an important category of chemicals that have a large volume of production and consumption around the world, industries such as detergents or chemical industries make greater use of chemical solvents. Some solvents are more important in industries due to their properties, Acetone and Xylene are among these solvents. In this blog, we will examine the properties and characteristics of these two important materials and generally, we will compare them.
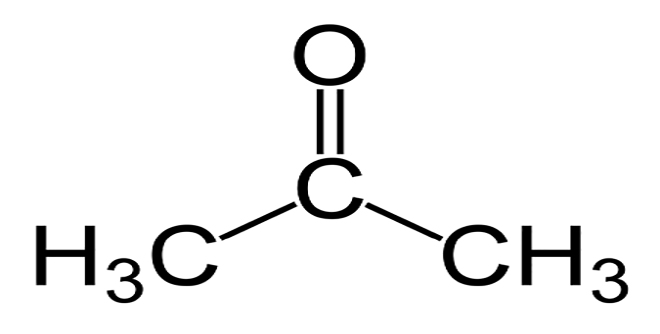
What is Acetone?
Acetone is very similar to xylene in terms of physical properties. It is an organic compound that has the appearance of a colorless liquid that, like xylene, this chemical is flammable and volatile. Acetone is a good solvent for organic compounds and it is also widely used in the polymer industry and the production of plastic products and synthetic fibers. It can also be used in the paint and resin industries and the production of adhesives, chemicals needed in agriculture, and the manufacture of drugs, this chemical can also be toxic, and all safety precautions must be taken when working with it.
What is Xylene?
Xylene is a fragrant, colorless, and flammable substance that has different isomers. The type of these isomers is determined based on the location of methyl functional groups on the benzene ring. A xylene is actually a group of benzene derivatives that have three isomeric structures of dimethyl benzene. The properties of this compound have led to its application in many industries. The role of this chemical as a solvent for various industries such as paints or as cleaners of various industrial colors is very common, when using this material, all safety and health should be observed because its long-term use can cause headache or dizziness.
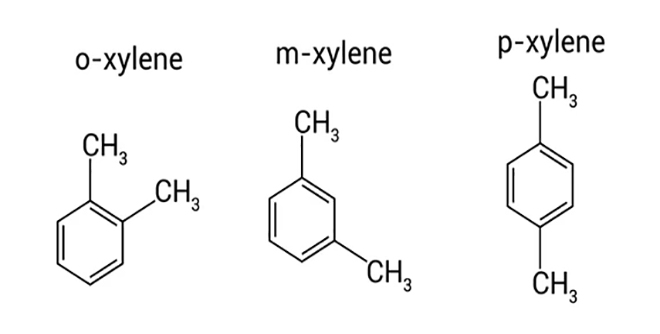
Is Xylene the same as Acetone?
The main commonalities of these two chemicals can be considered in their appearance and some of their physical properties. Their appearance, which is a colorless liquid, is due to the commonalities of these two substances, the high ability to dissolve various colors and organic compounds are other common denominators between these two substances.
Difference Between Xylene and Acetone:
Despite the many similarities in appearance, Xylene and Stone are different in many cases, the most important of these differences we will discuss below:
- The molar mass of acetone is about half that of xylene. Xylene can be found in the form of three different isomers whenever acetone is the simplest ketone available and it has no certain isomer. Xylene is considered non-polar in terms of polarity, but Acetone is slightly polar.
- In terms of the solubility of xylene, it can be dissolved lipophilic compounds, but hydrophilic and lipophilic acetone can be dissolved.
- Xylene is much more expensive and less toxic than acetone.
“The key difference between xylene and acetone is that xylene is a cheap and less toxic solvent, whereas acetone is an expensive and more toxic solvent. Moreover, xylene is nonpolar, and acetone is less polar; therefore, xylene can dissolve lipophilic substances, but acetone can dissolve both lipophilic and hydrophilic substances.”
Can you Use Acetone and Xylene to Clean Surfaces?
One of the important Common applications of xylene and acetone is in cleaning and polishing surfaces. Especially effective epoxy resins. Most people who deal with various resins, especially epoxy resins, use these two solvents to clean and remove the effects of resins.
Xylene and Acetone to Prepare the Paint:
In the paint and resin industry, the use of authorized additives for various purposes such as diluting, stabilizing, brightening, and finally creating suitable coatings on the created effect is very common. The use of solvents such as xylene and acetone helps to create stability, increase the useful life of paints and create freshness in paints.
Frequently asked questions
Can I use xylene instead of acetone?
Yes, because they are both great solvents for paint and ink. However, they are totally different compounds.
What solvent is stronger than acetone?
Methyl ethyl ketone, also known as MEK, is a solvent that is stronger than acetone but is very toxic.
What can I use instead of xylene?
Carrot oil, rose oil, or acetone are good alternatives to xylene. However, it is important to note that acetone is more toxic than xylene.
Which is better xylene or toluene?
Both of them are considered as a great oil remover however if we want to compare their influence, toluene is stronger than xylene.
What chemical can replace acetone?
Ethyl lactate is a chemical that can be used as a substitute for acetone. Actually, ethyl acetate is a great solvent for making perfumes.







6 Responses
Is xylene better than acetone?
The key difference between xylene and acetone is that xylene is a cheap and less toxic solvent, whereas acetone is an expensive and more toxic solvent.
Why is xylene a good solvent?
due to the fact it is a hydrocarbon, it is an excellent solvent for compounds that don’t dissolve in water. Xylene can dissolve hydrocarbons and many water-insoluble compounds.
What not to mix with xylene?
Xylene is not able to mix well with water