Description
Magnesium sulfate, also known as Epsom salt, is a chemical compound of oxygen, sulfur, and magnesium that occurs naturally in seawater and mineral springs. It is widely used in medicine, agriculture, and pharmaceutical industries.
Where to buy magnesium sulfate?
If you would like to know any further information about magnesium sulfate price and buying this chemical item, our team at Shanghai Chemex will be delighted to help you with that.
Chemical and physical properties
Magnesium sulfate appears as colorless crystalline powder. We can dissolve magnesium sulfate in water and release magnesium cation (Mg2+) and sulfate anion (SO4–). Some of the most important physical and chemical properties are listed below:
| Magnesium sulfate formula | MgSO4 |
| Density | 2.66 g/cm3 |
| Melting point | 1124 °C |
| Solubility in Water | Anhydrous form: 255 g/L |
| Heptahydrate: 710 g/L | |
| Molecular Weight | Anhydrous form: 120.36 |
| Heptahydrate: 246.47 | |
| Chemical structure | 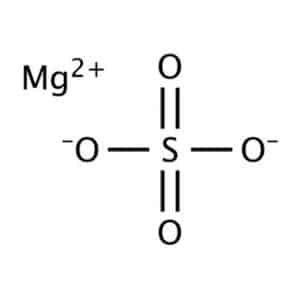 |
This chemical is soluble in boiling water, alcohol, and glycerin, but it can`t mix with acetonitrile. There are three types of magnesium sulfate:
- One water molecule (monohydrate)
- Seven water molecules (heptahydrate)
- No water molecules (anhydrous)
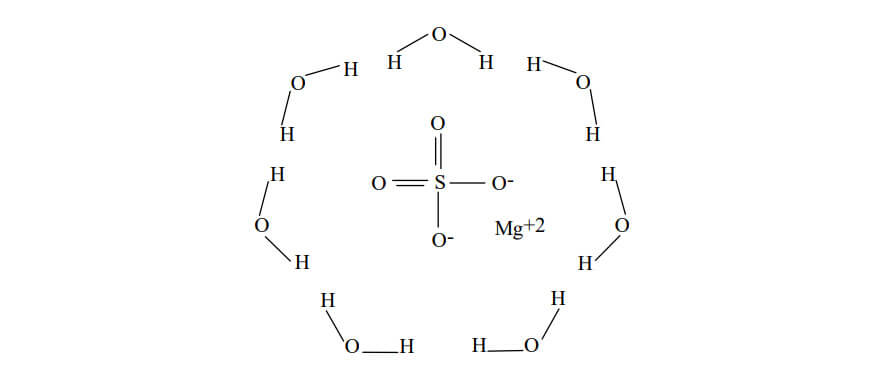
Here is the structure of magnesium sulfate heptahydrate:
The Production Process of Epsom salt:
Epsom salt can be prepared by reacting magnesium carbonate or magnesium oxide with sulfuric acid. Another method is to use industrial seawater or industrial effluents containing magnesium to precipitate magnesium hydroxide and react the precipitate with sulfuric acid.
MgCO3 + H2SO4 → MgSO4+ H2CO3
Magnesium Sulfate Uses:
Magnesium sulfate has a wide range of uses in agriculture, industry, food additives, animal feed, cosmetics, and medicine. In this section, we describe some of the applications of this chemical.
Agriculture
This compound is used in agricultural industries to prepare agricultural fertilizers to increase the amount of magnesium or sulfur in the soil. Potatoes, tomatoes, and peppers need magnesium-rich soil.
The solubility of this material is higher than other magnesium-based materials used for soil remediation. That is why it is used more.
Medical
In general, the human body needs a certain amount of magnesium (about 400 mg per day), which is why MgSO4 is used to control low levels of magnesium in the blood. Magnesium has an important role in cell function. If you have a well-balanced diet, you will get enough of it, and if you don’t get the required amount of this chemical substance, using magnesium sulfate can be a good choice. In some cases, it is also used to treat asthma, eclampsia, and stomach ailments.
- Magnesium sulfate in pregnancy
Doctors sometimes recommend magnesium sulfate in pregnancy because it reduces the risk of having seizures in pregnant women who have preeclampsia.
Chemical
Anhydrous magnesium sulfate, due to its structural properties, shows a great tendency to absorb moisture; in other words, it can be called a moisture absorber, so in the synthesis of organic matter in laboratories, it is used as a desiccant.
Bath salt
Magnesium sulfate’s mechanism of action in bath soap is that it is able to dissolve in water and release magnesium and sulphate ions. These ions are observed through the skin and help release muscle pain.
Magnesium sulfate toxicity
Some people with preeclampsia may benefit from taking magnesium sulfate. However suing extra amount of magnesium leaf to a situation which is known as magnesium overdose.
“Magnesium sulfate can be beneficial to some with preeclampsia. But there’s a risk of magnesium overdose, called magnesium toxicity. Taking too much magnesium can be life-threatening to both mother and child.”
Magnesium sulfate side effects
Using extra amount of magnesium sulfate might have side effects such as Confusion, muscle weakness and low blood pressure.
The bottom line
Magnesium sulfate, also known as Epsom salt, is widely used in the agricultural and medical industries. It has a large role in bath soaps and also as a desiccant. Although our body needs a significant amount of magnesium sulfate, using an extra amount has some side effects, such as confusion and muscle weakness.






Reviews
There are no reviews yet.