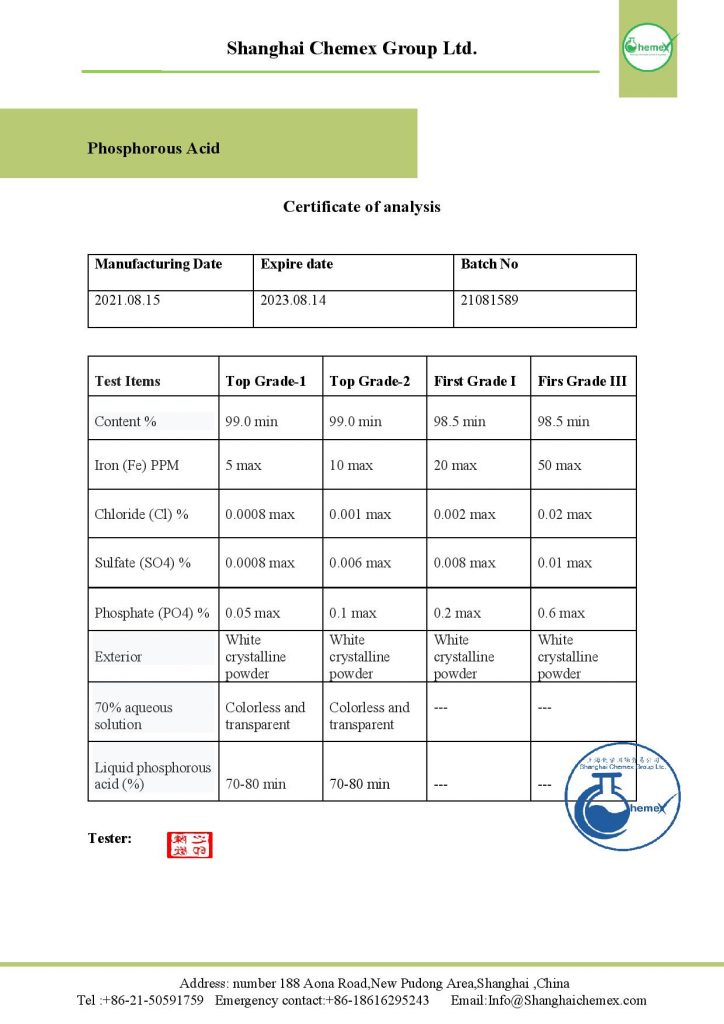
Description
Phosphorous Acid with the chemical formula H3PO3 is a chemical compound with polar molecules. This compound, also called orthophosphoric acid, is one of several oxygen-phosphorus acids used as a reducing agent in chemical decomposition. Alkali metal salts Phosphorous acid is widely used as an agricultural fungicide or as a superior source of plant phosphorus nutrition. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Phosphorous Acid is a compound with polar molecules that are seen in the form of a solid compound. This white and sometimes yellow crystalline product has a garlic-like taste. This compound with its acidic property dissolves well in solvents such as water; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical Formula | H3PO3 |
| Molecular Weight(g/mol) | 81.99 |
| Appearance | white solid |
| Taste | garlic-like taste |
| Density (g/cm3 at 21° C) | 1.651 |
| Melting Point (° C) | 73.6 |
| Boiling Point (° C) | 200 |
| Water Solubility (g/100 mL) | 310 |
| Solubility | soluble in water, ethanol |
| Color | white, colorless, or yellowish |
| Form | crystalline |
| Chemical Structure Depiction | 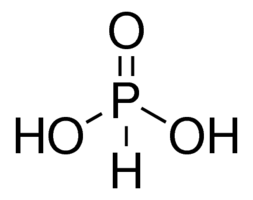 |
What is the Chemical Formula for Phosphorous Acid:
Phosphorous acid with the chemical formula H3PO3 is a polar molecular compound with a covalent bond between its constituent atoms. In this structure, phosphorus is the central atom, and two OH groups, one O atom, and one H atom are attached to it. The phosphorus atom uses sp3 hybrid orbitals to give the molecule a quadrilateral structure. Due to the electronegative difference between oxygen and phosphorus, as well as oxygen and hydrogen, most of the bonds of this molecule are polar, which makes phosphoric acid a polar molecule.
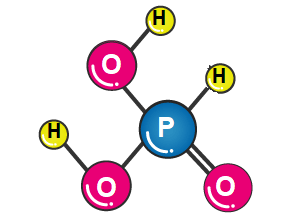
Difference Between Phosphorus and Phosphoric Acid:
Phosphorus and Phosphoric Acid are two types of acids that contain the chemical element phosphorus. The chemical structure of these two molecules is almost similar but their chemical and physical properties are different. The main difference between phosphorus and Phosphoric acid is that Phosphorus acid is diprotic while Phosphoric acid is triprotic.
Production Process of Phosphorous Acid:
Phosphorus acid can be prepared in several ways:
1. The reaction of phosphorus trichloride with water:
PCl3 + 3H2O → H3PO4 + 3HCl
The addition of PCl3 in the presence of concentrated Hydrochloric acid should be very cautious and slow.
2. By adding phosphorus trichloride to anhydrous oxalic acid:
PCl3 + 3(COOH)2 → H3PO3 + 3CO + 3CO2 + 3HCl
3. The main method of preparing Phosphorus acid is to dissolve P4O6 or tetra phosphorus hexoxide (diphosphorus trioxide) in water:
P4O6 + 6 H2O → 4 HPO(OH)2
Phosphorous Acid Uses:
- As a reducing agent in chemical analysis
- To prepare phosphate salts
- As a water softener, lime cleaner and products used in the process of collecting, purifying, and distributing water
- As an agricultural fungicide
- Insolvent extraction processes for the separation of heavy metals
- As a lubricant in industrial fluids such as hydraulic fluids
Is Phosphorus Acid Fertilizer Good for Plants?
Phosphorus is used in the form of Phosphorous acid in agriculture and as a repellent of toxins and pests. Because Phosphorus acid and its derivatives are not metabolized in plants, it is claimed that phosphonates can help phosphorus nutrients for plant growth and should be used with extreme caution.H3PO3 is an essential organic chemical for agricultural production that helps control the epidemic of agricultural fungi.

Buy Phosphorous Acid:
For more information on purchasing and ordering this product, please contact our experts in Shanghai Chemex through the numbers on the site.
Safety Information:
This chemical can damage the skin, eyes, mouth, and respiratory system. Because of the potential dangers of this chemical, it is important to use protective equipment when using it.
First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Handling and Storage:
Store in a cool, with proper ventilation, and keep away from incompatible substances such as oxidizing agents, metals, combustible materials, and alkalis.






SARA –
Why is phosphorous acid a weak acid?
china chemicals –
H3PO3 is a weak acid. Because it undergoes partial dissociation on dissolving in water or aqueous solution and produces a low amount of hydrogen ion. Lower the hydrogen ion in the solution, less is the strength of acidity of the compound.