Description
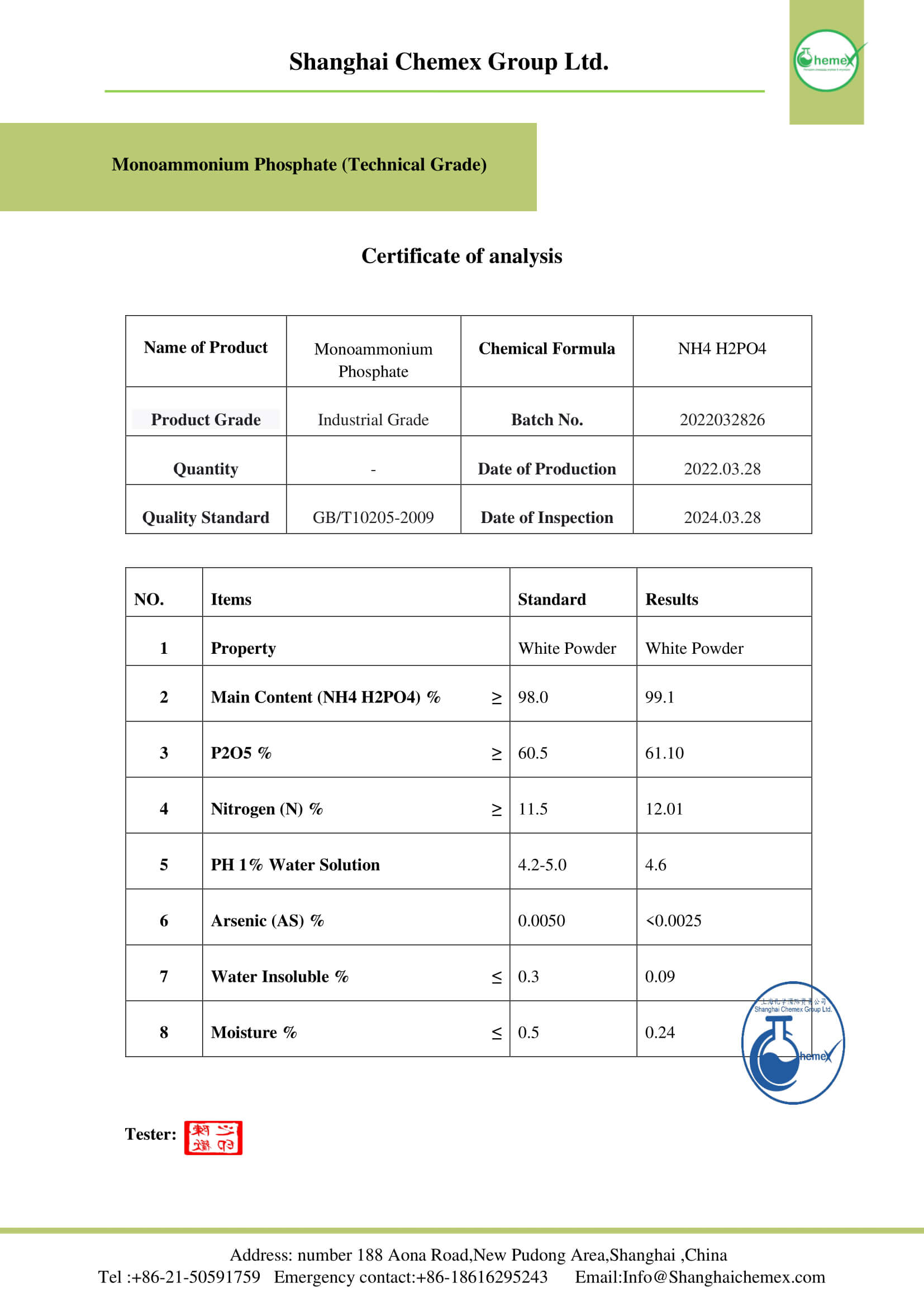
Monoammonium phosphate (MAP), also known as ammonium dihydrogen phosphate (ADP), is a chemical compound with the formula (NH4) (H2PO4) that is the main ingredient in agricultural fertilizers, and some fire extinguishers. It is also widely used in the optics and electronics industries. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.
Physical and Chemical Properties:
Monoammonium phosphate is available in powder or white tetrahedral crystals. This chemical compound is composed of ammonium cations [NH4] and dihydrogen phosphate anions [H2PO4] in equal proportions. This compound is soluble in water and almost insoluble in ethanol and stable up to 200 ° C; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | NH4H2PO4 |
| Molar mass (g/mol) | 115.03 |
| Appearance | solid |
| PH | 4.2 |
| Odor | Odorless |
| Density (g/cm3) | 1.80 |
| Melting Point (° C) | 190 |
| Solubility in water (g/100 g water at 25 °C) | 40.4 |
| Solubility | slightly soluble in alcohol; practically insoluble in acetone |
| Color | White |
| Form | crystals or powder |
| Synonyms | Ammonium dihydrogen phosphate, Ammonium biphosphate, mono ammonium salt |
| Chemical Structure Depiction | 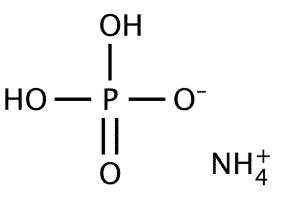 |
Monoammonium Phosphate Production Process:
Monoammonium Phosphate is industrially prepared by the exothermic reaction of phosphoric acid with ammonia in a suitable ratio and is initially crystalline and then precipitates.
- In this method, the raw materials are reacted in equal proportions, and MAP is produced, then the existing product is sent to a granulator and this material is produced in solid form.
- The second method consists of a cross tube reactor and the heat from the combination of Ammonia and Phosphoric acid causes the existing water to evaporate and the product to solidify.
NH3 + H3PO4 ↔ NH4H2PO4
As can be seen, the above reaction is a two-way reaction, That is, at high temperatures, the product decomposes and turns back into its constituents, namely ammonia and phosphoric acid.
Monoammonium Phosphate Uses:
- The highest use of mono ammonium phosphate in terms of weight is in agriculture as fertilizer. This compound provides the elements nitrogen and phosphorus in the soil in a form that can be used by plants. like potassium sulfate.
- Monoammonium phosphate is also a component of ABC powder in some dry chemical fire extinguishers.
- This material is a widely used crystal in the field of light due to its degradability. Crystals of this material are widely used in the optics industry due to their dual properties.
- This substance is used in its pure form as an animal feed additive. NH4 ion is synthesized and converted to protein, and the H2PO4 ion also supports many metabolic processes in animals.
What is Monoammonium Phosphate Fertilizer?
Monoammonium phosphate fertilizer is one of the well-known fertilizers in the agricultural industry. In fact, this fertilizer is the most widely used fertilizer in farms and the agricultural industry. The physical and chemical properties of ammonium phosphate fertilizer, including its rapid dissolution in water and relatively acidic pH, make it a suitable fertilizer for calcareous soils. Soil fertilizers are used to maintain soil fertility and productivity, prevent soil degradation and provide nutrients for crops and agriculture. The use of ammonium phosphate fertilizer is one of these cases. Chemical fertilizers provide the nutrients needed for plant growth. Plants grow fruits and crops by absorbing water and nutrients, carbon dioxide from the air, and energy from the sun. Apart from carbon, hydrogen, and oxygen, which together make up 90 95% of the dry matter of all plants, the other nutrients needed by plants are obtained mainly from the soils in which they grow. These essential nutrients are nitrogen, phosphorus, and potassium. Secondary nutrients are calcium, magnesium, and sulfur. In addition, plants need much smaller amounts of other nutrients. They need micronutrients including boron, chlorine, copper, iron, manganese, molybdenum, and zinc. Ammonium phosphate fertilizer (MAP) is an extensive source of phosphorus and nitrogen. These two elements are common ingredients in the agriculture and fertilizer industry. It also has the highest amount of phosphorus of any conventional solid fertilizer.
Buy Monoammonium Phosphate:
For more information on buying and ordering this product, please contact our experts in Shanghai Chemex through the communication channels available on the site.
Safety Information:
- Inhalation: High concentrations of dust in the air may cause scratching in the throat and cough.
- Eye and skin contact: Prolonged contact with dust may cause discomfort.
- Ingestion: Large quantities may cause disturbance of the gastrointestinal tract.
First-aid measures:
- Eye Contact: In case of contact with the eyes, immediately flush the eyes with plenty of water.
- Skin Contact: Remove this material from the skin with plenty of soap and water.
- Inhalation: Remove to fresh air immediately. If not breathing, give artificial respiration.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Handling and Storage:
Keep the container tightly closed in a cool, well-ventilated away from moisture.








MINA –
Is mono ammonium phosphate corrosive?
china chemicals –
Mono ammonium phosphate is slightly acidic in the presence of moisture resulting in mild corrosive properties. Monoammonium phosphate melts when heated above 300 °F (149 °C) forming a coating that will adhere to the surface.