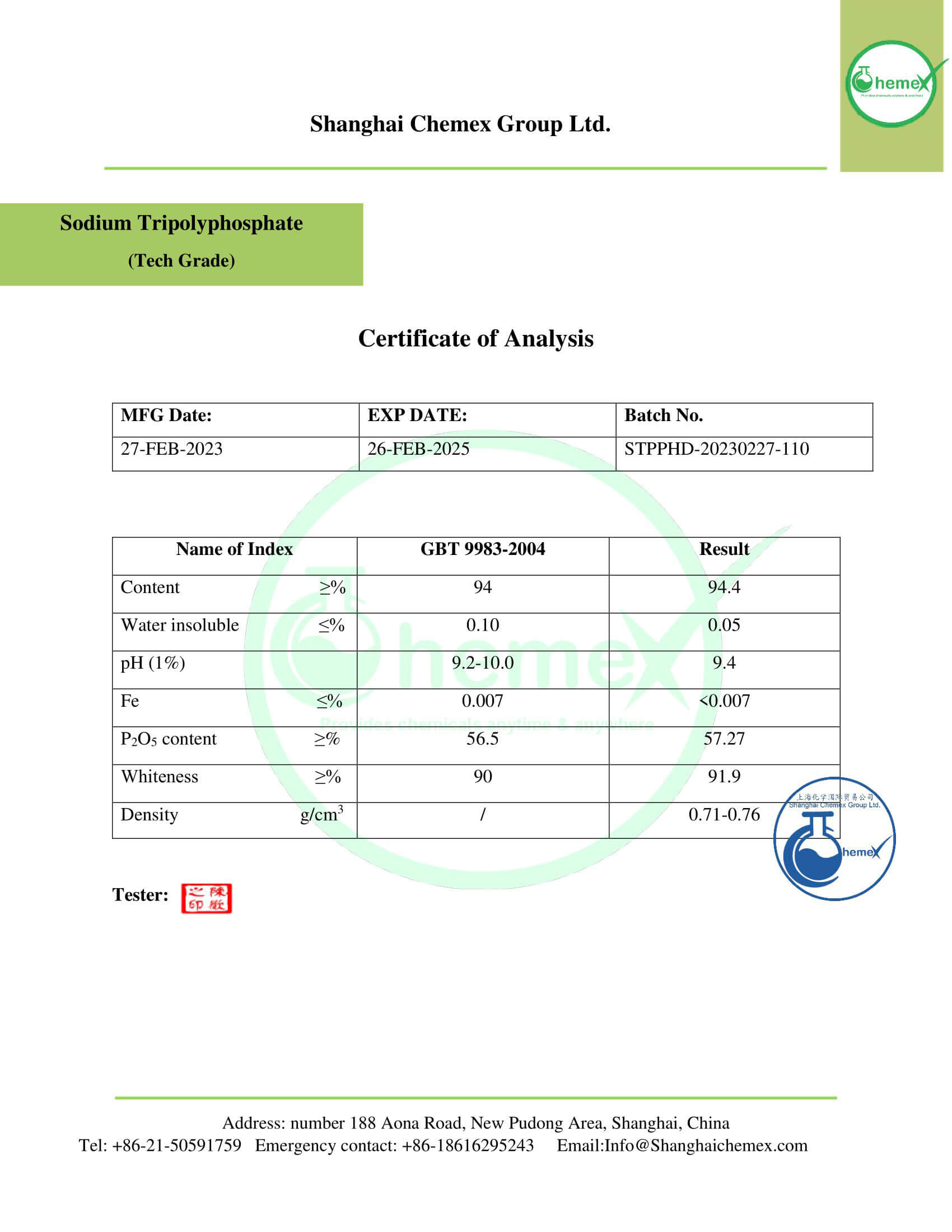
Description
Sodium tripolyphosphate (STPP) is a mineral chemical with the formula Na5P3O10. It is used as the main ingredient in detergents, cleaners, and soaps in various forms, including gels, liquids, and powders. Many household cleaning products, like surface cleaners and cosmetics, contain STPP.
How to buy sodium tripolyphosphate?
If you would like to know any further information about sodium tripolyphosphate’s price or buying this item, our team at Shanghai Chemex will be delighted to help you.
Physical and chemical properties
This compound appears as a white powder that is available in two forms: anhydrous and pentahydrate. Like other sodium salts, this compound is soluble in water, but it can’t mix with alcohols such as ethanol. In the table below, some of the chemical and physical properties of this product are mentioned:
| Chemical formula | Na5P3O10 |
| Molar mass(g/mol) | 367.864 |
| Appearance | white powder |
| Density(g/cm3) | 2.52 |
| pH | 9,1 – 10,2 |
| Melting Point(° C) | 622 |
| Solubility in water(g/100 mL) | 14.5 |
| Solubility | Insoluble in ethanol |
| color | White |
| form | crystalline forms |
| Chemical Structure Depiction | 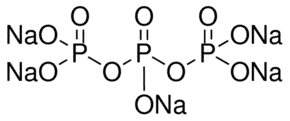 |
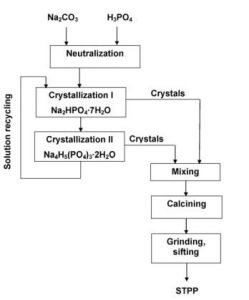
Sodium tripolyphosphate manufacturing process
The raw materials for the production of sodium are triphosphate, sodium carbonate, and phosphoric acid. To produce it, the first step is combining sodium carbonate and phosphoric acid, and after that, two salts called monosodium phosphate and disodium phosphate are produced. By combining these two salts together at a temperature of more than 300 °C, sodium tripolyphosphate is produced.
2 Na2HPO4 + NaH2PO4 → Na5P3O10 + 2 H2O
The second method is to use a wet process, the steps of which are as follows: The reaction of phosphoric acid with a sodium compound and the formation of a reaction mixture consisting of an aqueous solution of Na2HPO4 and NaH2PO4 with a pH above 6. Heat the reaction mixture to a temperature in the range of 400 to 550 °C and perform a crystallization step. Heat the compound at a high temperature, up to 700 ° C and then purify and finally produce a pure STPP product.
Use of sodiumtripoly phosphate
Detergents: Industries use STPP in detergents to wash heavy metals in water, such as calcium and magnesium, which causes the formation of insoluble metal salts, thus reducing the ability to wash or stop completely. Adding sodium tripolyphosphate to detergents can improve their efficiency.
- It produces more bubbles and foam and also increases the dispersing property.
- It can help remove stains and also soften the acid in water by neutralizing it
Foods: Additionally, some industries use sodium tripolyphosphate in food production as a preservative. Adding this salt to the meat, slows down the process of spoilage and provides freshness. STPP helps maintain the natural color of meat and improve its texture.
Water treatment: It can also be used in water treatment industries to remove heavy metals.
Mining: STPP is a cost-effective material that can be used in mining to enhance the flotation process of copper ore.
Self-care products: STPP is a great ingredient in mouthwash, shampoo, hair dyes, and skin care products.
Leather: Industries use STPP as a covering agent in the leather-making process.
Paper industry: It is also applied in the paper industry as a petroleum-resistant agent during paper printing.
Dyeing industry: Dying industries use it as an effective factor in preserving dye and preventing corrosion in textiles, rubber, fermentation, and antifreeze.
Sodium tripolyphosphate MSDS
The vapors of this compound are toxic and can cause allergies and redness when in contact with the skin and eyes. Also, excessive use of STPP may lead to symptoms such as vomiting, headache, abdominal pain, dizziness, and an irregular heartbeat. Therefore, people who deal with this substance should use special glasses, masks, and work clothes. They should store it in a cool place with proper ventilation and keep it away from incompatible substances.
The bottom line
Sodium tripolyphosphate is a useful additive that can increase the efficiency of detergents and make them clean stains much better. On the other hand, STPP has an important role as a food preservative. But if you are going to work with this chemical substance, pay attention to the safety information.
Frequently asked questions
What is sodium tripolyphosphate used for?
STPP is widely used in detergent formulations to increase their efficiency at washing stains. Moreover, this chemical substance has an important role in the food industry and water treatment.
What is sodium tripolyphosphate side effects?
In the case of inhaling STPP vapors, it can cause an allergic reaction and difficulty breathing. Also, if you use it without paying attention to the safety information, vomiting, headaches, and dizziness may occur.
What is sodium tripolyphosphate in shrimp?
STPP is a food additive that can be used to make the product last for a longer period of time. Also, it has an important effect on the color and appearance of the product.
Is sodium tripolyphosphate safe?
Yes, the FDA considers STPP a safe substance, but it’s important to note that people who are suffering from kidney disease should avoid using it.






arman –
What foods contain sodium tripolyphosphate?
china chemicals –
cured meat
deli meat
fast food
processed foods, such as ready-to-eat meals
commercially prepared baked goods and cake mixes
canned tuna
Olivia –
Why do industries add STPP to shrimp?
china chemicals –
STPP can increase the shelf life of shrimp. On the other hand, it improves the color and texture.