Description
Zinc oxide is an inorganic compound with the chemical formula ZnO, which is found in the form of a white powder and insoluble in water. This material is used in a wide range of different industries, including as an additive in the rubber industry, plastic. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Crude zinc oxide appears as odorless yellow-gray granules. This material has unique optical, electrical, and thermal properties that make it widely used in various industries. This inorganic acid can be used in electrically conductive applications because it is thermally resistant and resistant to extremely high temperatures. Due to its chemical and physical properties, it is widely used in the rubber industry. like Zinc stearate
The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | ZnO |
| Molecular Weight(g/mol) | 81.406 |
| Appearance | White solid |
| PH | 6.95 |
| odor | Odorless |
| taste | Bitter taste |
| Density (g/cm3) | 5.606 |
| Melting point (° C) | 1,974 (3,585 °F; 2,247 K) |
| Solubility | Soluble in acids and alkalies; insoluble in water and alcohol |
| Synonyms | Zinc white, calamine, philosopher’s wool, Chinese white, flowers of zinc |
| Color | White or yellowish-white |
| Form | powder |
| Chemical Structure Depiction | 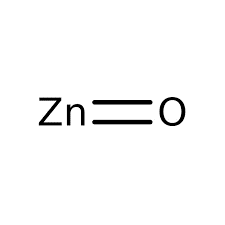 |
Structure of Zinc Oxide:
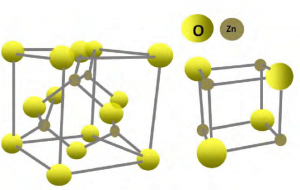
This inorganic oxide crystallizes in two main forms, hexagonal wurtzite and cubic zincblende. wurtzite structure is more stable in environmental conditions and is, therefore, the most common. In both structures, the zinc and oxide centers are tetrahedral.
Zinc Oxide Preparation:
Every year, thousands of tons of zinc oxide are produced in different ways and consumed in different fields. This combination is prepared in four general ways, and we will briefly describe each of these methods in the following.
Indirect method:
In the indirect method, zinc metal is melted in a graphite crucible and converted to steam at temperatures above 907 ° C. The steam reacts with the available oxygen to form a bright compound as the temperature drops. The oxide particles are then transferred to a cooling duct and collected in a chamber. Most of the zinc oxide produced in the world is produced indirectly.
Direct method:
In the direct method, zinc-containing rocks are melted and then the molten material is reduced to zinc vapors by heating by a carbon source such as anthracite. The difference between this method and the previous method is the degree of purity of the raw materials used. In the direct method, the final product has less purity and therefore lower quality.
Wet chemical process:
In this method, the process of producing zinc oxide from aqueous solutions of zinc salt begins. From these solutions, zinc carbonate or zinc hydroxide precipitate can be obtained, and then these solid precipitates are calcined at 800 ° C.
It is interesting to know that in the fourth method, which is laboratory synthesis, the color of zinc oxide can be changed to green or red by changing the concentration of oxygen used in the preparation of the final composition.
Zn + 2 H2O → Zn(OH)2 + H2
Zn (OH)2 → ZnO + H2O
Zinc Oxide Uses:
This material has different applications in various industries, including:
- Zinc oxide is used in the manufacture of many materials in the industry such as plastics, ceramics, glass, cement, rubber, paints, waxes, adhesives, foods, batteries, etc.
- In medicine and health, this compound called zinc oxide is used in baby powder, skin ointments, sunscreen, and anti-dandruff shampoo.
- Zinc oxide ointment is used in the treatment of skin lesions prone to infection such as burns, eczema, burns on the feet of infants, scratches and insect bites, mild anti-inflammatory skin, and also in combination with calamine as an anti-itch.
- This substance is one of the most important ingredients in cosmetics.
- This oxide is also used in the production of deodorants and soaps.
- This compound can absorb the sun’s UV rays and protect the skin from sunburn and other UV damage.
Why is Zinc Oxide used in the Rubber Industry?
Its physical and chemical properties make it one of the most important materials that are used as an additive in the rubber industry. the ZnO mixture with sulfur content leads to the production of a powerful vulcanization agent.
this material enhances the anti-aging properties of vulcanized rubber. In the procedure of rubber production, zinc oxide reacts with H2S which is released by polysulfide breaking bonds, and then new crosslinking bonds form. These phenomena lead to a more stabilized network and also improve the aging and thermal resistance of the final rubber.
The Effect of Zinc Oxide on Rubber Quality:
- Increase the rubber wear resistance.
- Improving tensile mechanical capability (increasing strength).
- Improve the thermal properties and increase the conductivity of rubber.
- Increase rubber life.
What is Zinc Oxide Used for in Ceramics?
The second important application of ZnO is in ceramics and also in special Tiles. The awesome heat capacity, thermal conductivity, and great temperature stability of this compound are enough reasons to use this material in this industry. Zinc oxide influenced the melting point and optical characteristics of the glaze. Based on its color, it has a complex effect on the final product.
Buy Zinc Oxide:
Shanghai Chemex is ready to provide services in the field of sales of zinc oxide with excellent quality, reasonable prices, and in desired quantities to customers. For information on the price and purchase of this product, you can contact our experts.
Safety Information:
- Harmful if swallowed, may irritate the digestive tract.
- Causes severe skin burns and eye damage.
- Prolonged inhalation of the dust may result in ever with symptoms of chills, fever, muscular pain, nausea, and vomiting.
- Very toxic to aquatic life with long-lasting effects.

First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
Store closed containers in a cool, dry, well-ventilated area.







Reviews
There are no reviews yet.