Corrosion is a natural phenomenon in which most of the atoms on the metal surface lose their electrons to the oxygen in the air or water. Actually, most of the metals are likely to do that. So it becomes an important concern for scientists, and they have conducted research on methods to prevent corrosion. Nowadays, there are several ways to inhibit or minimize corrosion attacks. One of them is using corrosion inhibitors.
What are corrosion inhibitors?
Corrosion inhibitors are chemical agents that protect metals by forming a thin layer. This coat can prevent the metal from rusting.
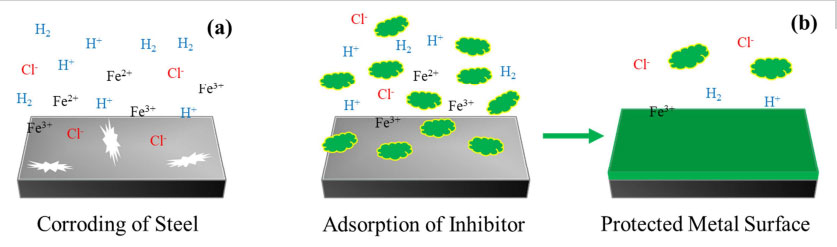
The mechanism of corrosion inhibitors is that they control and delay the undesirable phenomenon of corrosion by altering the interaction between the metal surface and the environment.
Types of corrosion inhibitors
Generally, there are five types of rust inhibitors:
1) Cathodic inhibitors
We make the metal surface the cathode in the electrochemical cell. So it doesn’t lose an electron, and the whole process of corrosion doesn’t take place. Actually, we turn the metal into a cathode to protect it. They can provide long-term protection. Sodium metabisulfite is an example of this kind of inhibitor.
Cathodic inhibitors can be used for water pipelines made of steel and also for ships and boats. In fact, these corrosion inhibitors are cost-effective.
“CP can prevent metal surfaces from corrosion. There are different types of CP; for example, galvanic protection or sacrificial protection, impressed current systems and hybrid systems.”
2) Anionic inhibitors (passivating inhibitors)
In this method, we can protect the surface by making it the anode of the electrochemical cell. One of the important advantages of anionic inhibitors is that they can protect complicated structures. And if we also want to point out disadvantages, we can say that they are one of the most costly inhibitors.
3) Volatile corrosion inhibitor (vapor phase inhibitors)
They are able to increase the pH of the atmosphere in order to control corrosion. For example, morpholine is used to control the corrosion of the condenser pipes in boilers.
4) Mixed inhibitors
These inhibitors reduce both anionic and cathionic reactions. For example, phosphates are corrosion inhibitors for water softening systems.
5) Green corrosion inhibitors
These chemicals are widely used in the oil and gas industries. Actually, they do not pollute the environment. Industries use them on different kinds of steel to minimize the corrosion attack.
Corrosion inhibitor applications
Many different systems, such as cooling systems, refinery systems, chemical operations, oil and gas production units, mainly use corrosion inhibitors. Additionally, they are widely used in fuel pipelines to make them last for a longer time. They can also be used as rust stoppers for cars.
The temperature and pressure range in tanks and pipes is high, and the inhibitor must be able to withstand this temperature and pressure and not lose its stability and efficiency in these conditions.
Corrosion inhibitors in the oil and gas industry:
As we mentioned, the oil and gas industry is one of the large industries that is mainly exposed to the undesirable phenomenon of corrosion, and the use of corrosion inhibitors in this industry is very practical and effective.
 In the oil and gas industry, the injection of low-ppm corrosion inhibitors such as amines and nitrites into the product stream prevents rust formation in the pipes. Corrosion of the outer walls of pipelines can be a very common issue, depending on the temperature, oxygen availability, and other factors. Cathodic protection is used to prevent the corrosion of oil and gas transmission pipes.
In the oil and gas industry, the injection of low-ppm corrosion inhibitors such as amines and nitrites into the product stream prevents rust formation in the pipes. Corrosion of the outer walls of pipelines can be a very common issue, depending on the temperature, oxygen availability, and other factors. Cathodic protection is used to prevent the corrosion of oil and gas transmission pipes.
The main cause of corrosion is usually oxygen. The inhibitory action that we can also use as a spray is hydrazine, which can convert oxygen into water. Generally, corrosion inhibitors are substances such as hexamine, phenylenediamine, dimethylethanolamine, and their derivatives. Of course, in these cases, sometimes antioxidants such as sulfite and ascorbic acid can be used.
Some corrosion inhibitors create an inactive coating layer by chemical adsorption on the surface. For example, benzotriazole is one of the chemicals used to protect the copper.
Corrosion prevention injection and sprays:
Nowadays, corrosion inhibitor injection packages as well as their sprays have made things easier for industries and factories. Using these packages, the materials used to prevent corrosion of the pipeline or storage tanks. It increases the life of pipelines, tanks, and equipment in a process and greatly reduces the cost of maintenance of these components, so that today the use of these packages and sprays has become a necessity.
Frequently asked questions
What is an example of a corrosion inhibitor?
We have five types of corrosion inhibitors. Among these classifications, morpholine and sodium metabisulfite are two common inhibitors.
What are the corrosion inhibitors for steel?
Green corrosion inhibitors are widely used to prevent steel corrosion in the oil and gas industries. Actually, they do not pollute the environment. Industries use them on different kinds of steel to minimize the corrosion attack.
Is EDTA a corrosion inhibitor?
Yes, EDTA can be used as a corrosion inhibitor. Moreover, we can name sodium benzoate as the other common inhibitor.
What is the corrosion inhibitor of carbon steel?
Alkanoamines and amines are good choices for preventing steel corrosion.
What are organic inhibitors of corrosion of metals?
They form a thin layer on the metal to control and delay the undesirable phenomenon of corrosion by altering the interaction between the metal surface and the environment.






