Description
Cobalt sulfate is a red, toxic, metallic salt used in the electrochemical industry, as a dryer in paints and inks, as a coloring agent, in storage batteries, and as a vitamin B12 supplement. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.
Physical and Chemical Properties:
If cobalt sulfate is dissolved in water, it is converted to cobalt cation and sulfate anion. Cobalt used in industry is mainly supplied from two materials, cobalt sulfate, and cobalt carbonate. The use of cobalt sulfate is more than cobalt carbonate. Cobalt oxide is less commonly used. red alum has interesting chemical and physical properties, for example, its melting point is higher than nickel or even iron alloy, it is weldable, and creates high heat resistance; The important physical and chemical properties of this material can be summarized in the table below:
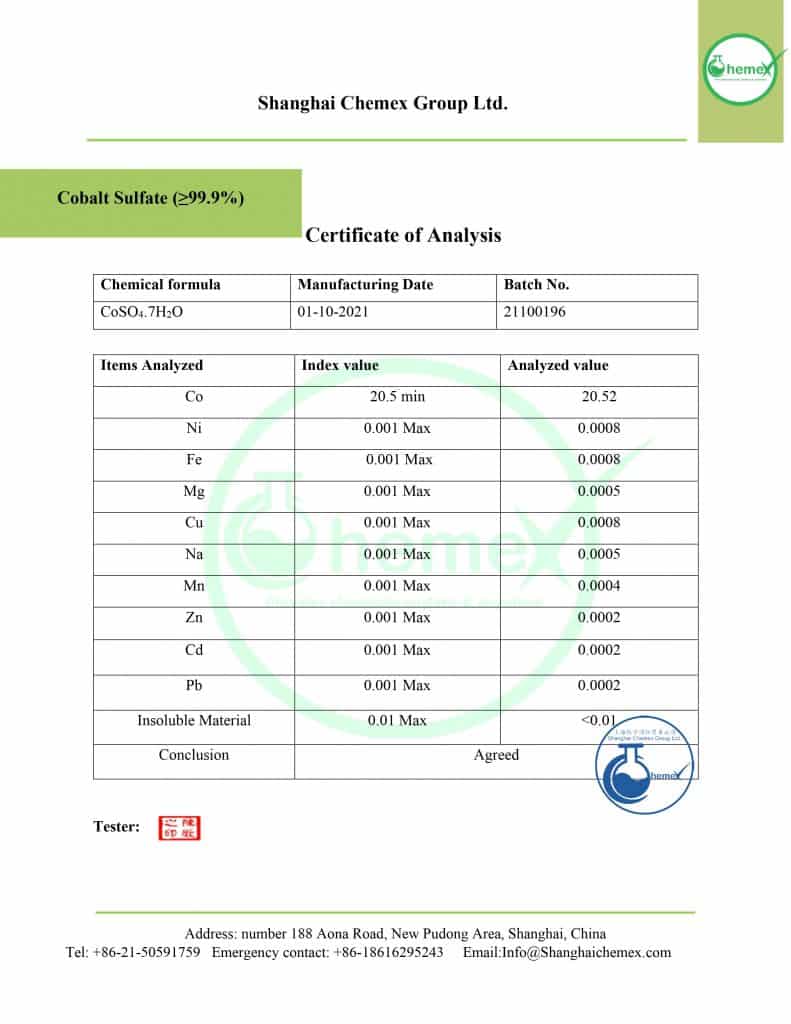
| Chemical formula | CoSO4 |
| Molecular weight (g/mol) | 154.996 |
| Appearance | reddish crystalline |
| Odor | Odorless |
| Density (g/cm3) | 3.71 |
| Melting point (°C) | 735 |
| Solubility in water (g/100 ml 25 °C) | 36.2 |
| Chemical Structure Depiction | 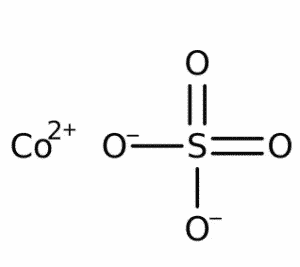 |
Production of Cobalt Sulfate:
red alum is produced in large quantities as a by-product during the production process of metals such as copper and nickel. Mainly in the production of this product, metal cobalt, sulfuric acid, and hydrogen peroxide are used.
Cobalt sulfate can be prepared professionally, like most sulfates, by dissolving cobalt metal in sulfuric acid. Or the compounds of carbonate, hydroxide, and cobalt oxide can be dissolved in sulfuric acid and prepared by crystallization and crystallization processes.
Cobalt Sulfate Uses:
According to the application, red alum is used in the manufacture of steel, very hard metal alloys (superalloys), magnets, hard materials, catalysts, paints, and batteries.
- Cobalt sulfate is used in the preparation of glass and ceramic paints. It is used in the preparation of pigments (blue and green) for ceramics and glass. These pigments are made from salts that are easily separable or from metal oxides. CoSO4 is mixed with other materials and ceramic-based materials and then heated.
- In medicine, this substance is used as a radioactive metal in radiation therapy (radiography). Cobalt is also used to treat certain types of anemia. To do this, cobalt sulfate is used to prepare vitamin B12.
- Superalloys are used in cases where the need for exceptional properties such as high mechanical strength, high-temperature resistance, corrosion resistance, and other harsh conditions is felt. Cobalt-based super-alloys are used in jets and other applications as well as Gas turbines Rocket engines, Nuclear reactors, Power plants, and Chemical equipment.
- Most of this material is used in the manufacture of batteries, especially batteries used in electric vehicles. Cobalt is one of the main elements in the manufacture of lithium batteries because the use of cobalt as a cathode in this type of battery significantly increases Energy, power, and performance improvement compared to cobalt-free batteries They are also used in the synthesis of polyester products and the production of aldehydes and other industrial reactions. To create glossy coatings on nickel, this compound can be added to the plating bath.
Other uses :
- red alum is mainly used as a catalyst to remove sulfur from petroleum products.
- Cobalt sulfate can prevent the release of sulfur contaminants into the air by removing sulfur during the sulfurization reaction. As a result, with the help of this compound, sulfur can be removed from natural gas and refined petroleum products such as gasoline, diesel, kerosene, and fuel oils that are used in vehicles, aircraft, and ships.
Safety:
Research on red alum has shown that its respiration can be harmful to the respiratory system and can cause serious damage to the lungs, heart, liver, and kidneys. Its direct contact with the skin of the body causes irritation and itching. we must observe safety and preventive measures such as the use of gloves and special clothing When working with this material.

Packing and Storage:
This compound is better to store cobalt sulfate away from moisture and direct sunlight in a cool, dry place.







Reviews
There are no reviews yet.