Description
Cobalt oxide is an inorganic compound whose crystals are dark red, olive green, gray, or black. Cobalt oxide particles are important magnetic materials and are p-type semiconductors. This compound is most used in the ceramic industry and is used for blue glazes and enamels. Shanghai Chemex is ready to provide services in the field of supply and sale of chemicals required by industries and manufacturers.
Physical and Chemical Properties of Cobalt Oxide:
This powder compound with the chemical formula of CoO is an odorless, tasteless powder that is insoluble in water but soluble in hot mineral acids; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | CoO |
| Molar mass (g/mol) | 74.9326 |
| Appearance | powder |
| Density (g/cm3) | 6.45 |
| PH | 5-7 |
| Odor | odorless |
| Melting Point (° C) | 1,933 (3,511 °F; 2,206 K) |
| Solubility in water | insoluble in water |
| Color | olive or gray |
| Form | crystals |
| Chemical Structure Depiction | 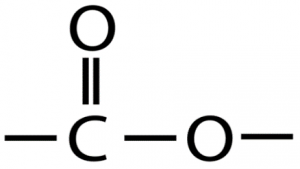 |
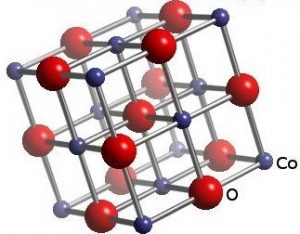
The chemical Structure of Cobalt Oxide:
Cobalt oxide is a mineral compound with the molecular formula of CoO and the structure of this compound is in the form of a typical spinel structure. Each cubic unit cell contains four Co2+ and four O2− ions.
Cobalt Oxide Soluble or Insoluble:
Cobalt oxide crystals are insoluble or partially soluble in water but are highly soluble in acids and alkaline solutions. It is also insoluble in ammonium hydroxide and soluble in acidic and alkaline hydroxides.
Production of Cobalt Oxide:
- This substance is usually obtained by heating cobalt hydroxide precipitated from sodium hypochlorite solution. The reaction equation is shown in the figure below:
Co (OH) 2 → CoO + H2O
- Other ways of producing this material include controlled oxidation of cobalt metal at temperatures above 900 ° C and cooling it at room temperature and controlled. This powder composition is prepared for the thermal decomposition of cobalt nitrate or sodium carbonate.
2Co3O6 → 6CoO + O2
Cobalt Oxide Uses:
- As a catalyst and oxide agent in the chemical industry.
- Production of coal raw materials.
- As a color in glass and ceramics. like chrome oxide

- Use in hard alloys.
- As a pigment.
- animals feed and fertilizer.
- production of cobalt (II) salts.
- In temperature and gas sensors.
Cobalt Oxide in Medicine:
Cobalt oxide although it has a wide range of applications in the production of superconductors, semiconductors, sensors, MRI, and biomedicine, it’s a nanoscale biological synthesis with a completely green method for the production of cobalt oxide nanoparticles is a major achievement in the medical sciences. The nanoparticles of this material show different antibacterial potentials against different types of pathogenic bacteria. These compounds are more compatible with red blood cells than macrophages and have antioxidant, regenerative, and enzyme inhibitory properties.
Cobalt Oxide Nanoparticles:
Recently, special attention has been paid to preparing Co3O4 with nanostructure and improving its properties, so that separate nanostructures such as films, tubes, fibers, and rods have been made from this material. Cobalt oxide is often used for catalytic and magnetic applications. One of the important applications of cobalt oxide nanoparticles:
- In medicine, especially for drug delivery and magnetic resonance imaging (MRI).
- Biological sensors.
- Magnetic memories
Buy Cobalt Oxide:
For information on how to purchase this product and place an order, you can contact our partners at Shanghai Chemex.
Safety Information:
Cobalt oxide is classified as a dangerous substance. This product can be toxic to all organs of the body if swallowed and exposed to inhalation. It can also be irritating to the skin and mucous membranes of the body.


Packing and Storage:
Store in a dry and cool place with proper ventilation in a tightly closed container.







Reviews
There are no reviews yet.