Description
Ferrous sulfate heptahydrate or Iron (II) sulfate is a compound with the chemical formula FeSO4.7H2O, which depending on the pH can be produced as blue or green crystals. Normally, seven molecules of water are present in the composition of ferrous sulfate, and if dried, it can lose six molecules of water and turn into a white, single water powder (monohydrate). This compound is widely used in various industries such as agriculture, pharmaceutical, food, and laboratory. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.
Physical and Chemical Properties:
Iron (II) sulfate is an inorganic compound with a crystalline structure, and the higher the moisture content, the larger the crystals will be. In terms of color, this combination is light green or bluish-green; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | FeSO4.7H2O |
| Molecular Weight(g/mol) | 278.02 |
| Appearance | solid |
| odor | Odorless |
| Density (g/mL) | 1.895 |
| Melting point (° C) | 60–64 |
| Water Solubility (g/100 mL 25 °C) | 29.51 |
| Synonyms | Iron (II) sulfate; Ferrous sulfate, Green vitriol, Iron vitriol |
| Color | Blue-green |
| Form | crystals |
| Chemical Structure Depiction | 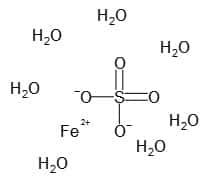 |
Ferrous Sulfate Production Process:
Ferrous sulfate is produced by dissolving iron filings in dilute sulfuric acid or oxidizing wet iron sulfide in the presence of air.
Fe + H2SO4 → FeSO4 + H2
It is also produced as a by-product in steel acid washing units and in the titanium oxide production process. This product is obtained from the liquid flow of acid washing units in an almost pure form. The purity of ferrous sulfate obtained from the titanium oxide production process depends on the type of ore concerned.
2FeS2 + 7O2 + 2H2O → 2FeSO4 + 2H2SO4
Iron (II) Sulfate Uses:
- In paint production industries such as iron pigments
- As raw materials for the production of agricultural fertilizers
- In the feed industry of poultry as an iron vitamin
- As a catalyst in the chemical industry
- In the tanning and leather industries
- As a precursor to the synthesis of other iron compounds
- In water treatment industries to improve water and wastewater
Is Ferrous Sulfate Good for Plants?
The chemical composition of ferrous sulfate is used to produce a variety of fertilizers. This substance is added to agricultural soils to eliminate soil iron deficiency and supply plant nutrients. Iron is essential for plant growth, especially for germination, flowering, and chlorophyll production. Soil iron deficiency occurs for a variety of reasons; Increasing the alkalinity of the soil, inadequate irrigation, loss of proper ventilation for the soil, and increasing the amount of carbon dioxide in the soil environment are factors that reduce the amount of iron required by the plant in the soil. This is the best time to use ferrous sulfate fertilizers.

Among the advantages of this iron fertilizer is its very reasonable and cheap price compared to iron chelate fertilizer. Also, iron sulfate fertilizer contains sulfate ions, which indicates the presence of sulfur in it. Sulfur, in addition to being the fourth essential element needed by plants and trees, on the other hand, it reduces soil pH and soil salinity of agricultural lands and facilitates the absorption of micronutrients and essential elements by plants.
What are the Medicinal uses of Ferrous Sulfate?
Iron deficiency is the most common nutritional deficiency in the United States, affecting athletes, young women, vegetarians, and the elderly more than anyone else. Iron (II) sulfate, along with other iron compounds in foods, is used to treat low levels of iron in the blood caused by conditions such as pregnancy or anemia. Iron helps transport oxygen through the blood to the human body. like Ferrous Sulphate Anhydrous
Global Market:
Ferrous sulfate is one of the most widely used chemicals. Germany and the United Kingdom in Europe India and China in Asia are the major global producers of this substance. Of course, the production method of developed countries is different from the third world, so that they are produced and concentrated from the effluents of large steel mills, for example, in China, the abundance of minerals such as titanium oxide is used.
Safety Information:
The combination of ferrous sulfate is not toxic or dangerous in itself, but like other chemical compounds, safety and caution must be exercised. When working with this combination, it is better to use gloves, glasses, and a mask
Packing and Storage:
This compound must be stored in a dry environment, completely closed, and at a temperature of +15 to +26 degrees.







Reviews
There are no reviews yet.