Description
Ferrous Sulphate Anhydrous, also known as Iron(II) sulfate, is a salt of iron with the chemical formula FeSO4 This product can be produced as blue or green crystals depending on the PH. Normally, there are seven molecules of water in the composition of ferrous sulfate, which, if dried, loses six molecules of water and turns into a white watery powder. Ferrous sulfate is one of the raw materials for chemical fertilizer production. The amount of ferrous sulfate used in cereals and fruit trees varies depending on the type of crop and the type of soil. For example, it has the highest amount of pistachio trees in saline areas. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.
Physical and Chemical Properties:
Ferrous Sulphate is a green compound with the formula FeSO4, which is produced in a light green color, but due to its properties, it can also change color to bluish-green, white, yellow, and brown. It is initially produced as hydrated crystalline Iron Sulphate, which contains a large amount of moisture. As the fluid in it decreases, the compound disappears. The drier the Ferrous Sulfate, the whiter it’s color; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | FeSO4 |
| Molecular Weight(g/mol) | 151.91 |
| Appearance | solid |
| odor | Odorless |
| Density (g/cm3) | 3.65 |
| Melting point (° C) | 680 (1,256 °F; 953 K) |
| Water Solubility | Soluble in water |
| Other names | Iron (II) Sulphate; Ferrous sulfate, Green vitriol, Iron vitriol, Copperas, Melanterite, Szomolnokite |
| color | White crystals |
| form | crystals |
| Chemical Structure Depiction | 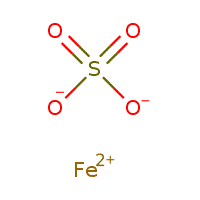 |
Production Process of Ferrous Sulphate Anhydrous:
Ferrous Sulphate is produced by dissolving iron filings in dilute sulfuric acid or oxidizing wet iron sulfide in the presence of air. It is also produced as a by-product in steel acid washing units as well as in the titanium oxide production process. This product is obtained from the liquid flow of acid washing units in almost pure form. The purity of Iron(II) Sulphate obtained from the titanium oxide production process depends on the type of ore concerned.
Ferrous Sulphate Uses:
Ferrous Sulphate Anhydrous is a chemical used in agriculture, industry, and medicine.
- Like aluminum sulfate, Ferrous Sulphate is used as an agrochemical to lower the pH of highly alkaline soils. Therefore, plants can easily access soil nutrients. In horticulture, it is used to treat jaundice of plant leaves caused by iron deficiency and has many lasting effects.
- It is used as a coagulant to combine with impurities and contaminants in water treatment and their sediment to produce drinking water. Ferrous Sulphate can absorb heavy ions, oils, and phosphorus in water and is used for decolorization in various industrial effluents, including printing, dyeing, and plating.
- Along with other iron compounds, Iron Sulphate strengthens foods and treats iron levels in the blood caused by anemia or pregnancy. Iron helps the blood carry oxygen in the human body.
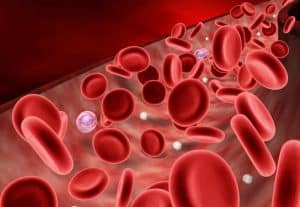
- Ferrous Sulphate is a reducing agent and is used in cement to reduce chromate to chromium compounds which are less toxic.
- Today, Ferrous Sulphate is used as a dye in food and fabric dyes. This compound produces a variety of colors. Such as olive green, crimson-purple, gray, and chocolate brown.
Buy Ferrous Sulphate Anhydrous:
For more information on purchasing and ordering this product through the numbers available on the site, please contact our experts in Shanghai Chemex.
Safety Information:
Ferrous Sulphate is a type of iron that is commonly found in the foods you eat. The human body needs iron to make blood, and iron sulfate is an essential mineral for the body that is used as a medicine to treat anemia. Iron is an essential heavy metal found in many multivitamin supplements and is used therapeutically in higher doses to treat or prevent iron deficiency anemia. When taken in excess of the recommended daily allowance or in the form of alternative doses, it can have adverse effects on the liver. Intentionally or accidentally consuming too much iron can cause serious poisoning, one of the side effects of which is acute liver damage. Research has shown that high iron intake, especially through supplements, can produce free radicals in the colon, leading to cell damage and eventually cancer.
The main danger of this compound is for the environment, this substance is used for water and wastewater treatment as well as fertilizer and it should be prevented from spreading in the environment by taking serious measures.
Packing and Storage:
The material should be stored in a tightly-closed container in a cool, dry, well-ventilated area. Keep product away from heat sources and direct sunlight.







Reviews
There are no reviews yet.