Description
Acrylic acid is the simplest unsaturated carboxylic acid that has a vinyl group attached to the end of the carboxylic acid. The appearance of this substance is a colorless and clear liquid and has the chemical formula CH2 = CHCOOH. This combination is also known as propene acid, ethylene carboxylic acid, and propenoic acid. The main uses of acrylic acid are in the production of acrylic esters and acrylic resins, which are used in the manufacture of adhesives and coatings. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Acrylic acid is a colorless, corrosive liquid with a pungent odor that is soluble in water, alcohol, ether, benzene, chloroform, and acetone. It is also sensitive to heat and direct sunlight. This material is very unstable and tends to polymerize. The famous properties of acrylics are good conductivity of light, color, and dimensional stability; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | C3H4O2 or CH2CHCOOH |
|
Molecular Weight(g/mol) |
72.063 |
| Appearance | Clear, colorless liquid |
| Odor | Acrid |
| Density(g/cm3) | 1.051 |
| Melting Point(° C) | 14 |
| Boiling point(° C) | 141 |
| PH | 3 |
| Solubility in water | Miscible |
| Color | Colorless |
| Form | Liquid |
| Chemical Structure Depiction | 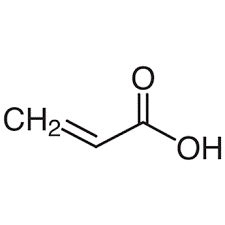 |
Formula and Structure of Acrylic Acid:
Acrylic acid consists of a vinyl group connected directly to a carboxylic acid terminus with the formula CH2=CHCO2H. The chemical structure of acrylic acid is such that it facilitates the combination with a large chain of polymer constituents.
Acrylic Acid Production Process:
Acrylic acid is produced from the oxidation of propylene, which is a by-product of the production of ethylene and gasoline.
2 CH2=CHCH3 + 3 O2 → 2 CH2=CHCO2H + 2 H2O
In the past, acrylic acid was also produced by hydrolysis of acrylonitrile, but this happened when propane was oxidized. This production process was generally abandoned because it produced ammonium by-products, which had to be separated. Acrylic acid monomers are produced by the reaction of acetylene, carbon monoxide, and alcohol in the presence of a nickel catalyst. In this reaction, if water is used, acrylic acid is obtained.
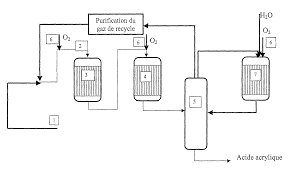
Another method of producing this acid is the catalytic oxidation of propylene with acrolein. Oxidation of propylene produces acrylics, acrylic acid, acetaldehyde, and carbon monoxide. Acrylic acid is obtained from the initial oxidation while acrolein is produced in the second stage. Azeotropic distillation is used for the final purification process.
Acrylic Acid Uses:
Acrylic acid is used in many industries such as water purification, textiles, diapers, detergents, cosmetics, packaging, leather, food, oil and gas, paper, coatings, adhesives, and paints.
- The main application of acrylic acid is in the production of acrylic esters and acrylic resins, which are used in the manufacture of adhesives and coatings.
- It is also used in the production of petroleum chemicals, detergents, and water-absorbing acrylic polymers.
- This compound is a monomer of polyacrylic acid and polymethacrylic acid. Another application is in polymer solvents for the production of acrylic paints and coatings. like Isothiazolinone

Other applications:
- Production of polyelectrolytes.
- It is used as an antibacterial agent.
- Production of cosmetic products such as nail polish.
- In the manufacture of rubber.
- In pharmacy for the production of tablet coatings.
Buy Acrylic Acid:
Contact our experts in Shanghai Chemex to find out how to place an order and find out the price of this product and buy and sell industrial acrylic acid.
Safety Information:
- Constant exposure to this acid irritates the skin and respiratory system, while exposure to small amounts is generally not harmful to the body.
- High doses of this acid can be toxic and lead to lung problems in humans.
- Concentrated solutions of acrylic acid, if swallowed, can cause severe burns in the mouth and gastrointestinal tract.
- In addition, its acidic vapors can disrupt the mucous membranes and respiratory system.
- It is very toxic to aquatic life.
- This compound is also flammable.
First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and storage:
Keep containers tightly closed in a dry, cool, and well-ventilated place. Keep away from heat and direct sunlight. The best storage temperature for this material is 15 to 25°C.









Reviews
There are no reviews yet.