Description
EDTA 4Na with the chemical formula C10H12N2O8Na4 is obtained by neutralizing ethylene diamine tetraacetic acid with 4 equivalents of sodium hydroxide. Various applications of this compound include water purifiers, disinfectants, sanitary detergents, etc. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.
Physical and Chemical Properties:
Tetrasodium EDTA is a solid, colorless, and water-soluble amino polycarboxylic acid that is used in various industries and is mostly used as a chelator and inactivates them by binding to specific mineral ions; The following table lists some of the important chemical and physical properties of this compound:
| Chemical formula | C10H12N2Na4O8 |
| Molecular Weight(g/mol) | 380.171 |
| Appearance | solid |
| PH | 11.3 |
| Melting point (° C) | 300 |
| Solubility | Freely soluble in water, Slightly soluble in ethanol |
| Color | white |
| Form | crystals or powder |
| Chemical Structure Depiction | 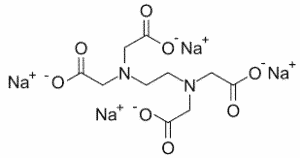 |
Production Process Of EDTA 4Na:
EDTA 4Na was first produced by Munz in 1935 from a mixture of ethylenediamine and chloroacetic acid. But today it is mainly obtained from ethylenediamine, formaldehyde, and sodium cyanide. In this way, sodium salt is formed, which subsequently turns into acid in the next steps.
- H2NCH2CH2NH2 + 4CH2O + 4 NaCN + 4 H2O → NaO2CCH2)2NCH2CH2N(CH2CO2Na)2 + 4NH3
- (NaO2CCH2)2NCH2CH2N(CH2CO2Na)2 + 4 HCl → (HO2CCH2)2NCH2CH2N(CH2CO2H)2 + 4NaCl
This process is used and exploited every year to produce 80,000 tons of EDTA in the world.
Tetrasodium EDTA Uses:
Tetrasodium EDTA is mostly used in the production of detergents. Water and wastewater treatment and the paper industry are in the next categories.
Industrial Detergents:
Hard water is the main challenge of detergents in the cleaning process. Hard water is water that has high amounts of metal ions and ionic compounds. Detergents normally react with these metal ions and ionic compounds in water and lose their properties. The purpose of using EDTA is quite simple; Prevent the presence of minerals in the water from the reaction with detergents and thus perform a perfect cleaning process even in hard water.
Water Treatment:
Water and sewage pollution from industrial and domestic activities has become a serious problem. Advances in industries such as mining, steel, and metal plating have allowed water contaminated with harmful metals to enter nature. These dangerous elements enter the human food chain through direct drinking of polluted water or through plants and animals, causing irreversible effects on the human body. For this reason, the use of chelating compounds, led by Tetrasodium EDTA, has received more and more attention. EDTA 4Na is widely used in water and wastewater treatment due to its abundance and cost-effectiveness. This compound can absorb and neutralize metal ions such as lead, cadmium, mercury, and cobalt. The combination of EDTA and metal ions is then removed from the water by methods such as reverse osmosis, and the purified water returns to nature or the cycle of use.
Paper Industry:
EDTA contributes to the whiteness and luminosity of paper by combining it with metal ions to catalyze the decomposition of hydrogen peroxide in the paper-making process. This compound also blends with wood lignin in pulp to give the paste a shiny, white appearance. like EDTA 2Na
Buy EDTA 4Na:
Tetrasodium EDTA is one of the useful compounds in various industries such as detergent production, and water treatment. To register your order and find out the price, you can contact our experts in Shanghai Chemex.
Tetrasodium EDTA Hazards:
This substance has many environmental problems. Because its lifespan is very high and different species of Tetrasodium EDTA are very stable in the environment and do not decompose very easily. Even in the presence of sunlight, their decomposition proceeds very slowly. Excessive use of this substance can cause nausea and vomiting, lower blood pressure, liver damage, and skin allergies.


First-aid measures:
- Eye Contact: In case of contact with the eyes, immediately flush the eyes with plenty of water.
- Skin Contact: Remove this material from the skin with plenty of soap and water.
- Inhalation: Remove to fresh air immediately. If not breathing, give artificial respiration.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
This compound should be stored in a cool place with proper ventilation and away from direct sunlight because it will release toxic and irritating gases in case of ignition.










Reviews
There are no reviews yet.