Description
Hydrazine hydrate is a colorless compound with the chemical formula H6N2O, which is used as a reducing agent in the synthesis of many reactions and as a solvent for many mineral compounds. Generally, hydrazines are a group of organic substances that are obtained by the replacement of one or more hydrogen atoms by an organic group. This compound is obtained by some microorganisms such as bacteria, yeast, and fungi. Hydrazine, like ammonia, is a chemical base but is 15 times weaker than ammonia. This compound is used in the synthesis of many chemicals, water treatment, satellite fuel, medicine making, etc. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Hydrazine Hydrate is a colorless substance with an ammonia-like odor and is found in both industrial and health grades in the market; In the table below some of the chemical and physical properties of this product are mentioned:
| Chemical formula | H6N2O |
| Molecular Weight(g/mol) | 50.061 |
| Appearance | Clear Liquid |
| odor | Ammonia-like |
| Density (g/cm3 at 25 °C) | 1.03 |
| Melting point (° C) | -57 |
| Boiling point | 120.1 |
| Water Solubility (g/100 cc) | Miscible |
| Other names | Hydrazine Monohydrate; Hydrazine Hydrate Solution; Hydrazinium Hydroxide; Hydrazine Hydroxide |
| color | Colorless |
| form | oily liquid |
| Chemical Structure Depiction | 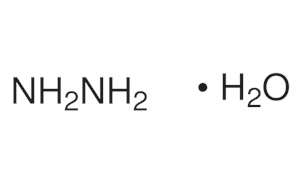 |
The Production Process of Hydrazine Hydrate:
Commercially, this compound is produced in three ways:
Rashing Process:
Ammonia is oxidized by chlorine amine and sodium hypochlorite. Chloramine then reacts with excess sodium hydroxide and ammonia to produce an aqueous solution of hydrazine or hydrazine hydrate. During this reaction, sodium chloride is produced as a by-product.
Ketazine Process:
This process involves the oxidation of ammonia with hydrogen peroxide in the presence of nitrile or an amide group to form an azine that is further hydrolyzed to produce hydrazine hydrate.
Peroxide Process:
In this process; hydrogen peroxide is used to oxidize ammonia in the presence of ketones.
Hydrazine Hydrate Uses:
This material is used for many industrial applications including the Preparation of polymeric foam, polymerization catalysts, pesticides, and the gas used in airbags.
- For the production of antituberculosis drugs and anti-diabetic drugs. Used in rocket fuels, power plants, and fuel cells as a safer alternative to hydrogen.

- Used in medical materials, pesticides, pigments, coolants, and photographic developers.
- Mainly used for detoxification of AC, D1PA, and TSH foam materials.
- It can be used to produce herbicides, plant growth regulators, insecticides, sterilizers, etc.
- As an intermediate in the synthesis of organic matter (chemicals, pharmaceuticals, etc.)
- Corrosion inhibitors in industrial boilers, nuclear or thermal power plants
- Inhibition of heavy metals, lime, and pollutants such as Br2, Cl2, and NOx in the water treatment process.
Safety Information:
- It explodes at temperatures above 38 ° C due to heat, flame, or ultraviolet radiation.
- Inhalation of this substance causes symptoms such as headache, nausea, sore throat, convulsions and burning sensation, and severe cough.
- Hydrazine Hydrate is in contact with corrosive skin and causes redness, pain, and burns to the skin. To prevent this accident, gloves and protective clothing must be used.
- If this compound comes in contact with the eyes, it causes redness, corrosion, paint, and deep and serious burns. To prevent this from happening, use eye protection or special glasses.
- Dangers of swallowing and taking hydrazine Hydrate include severe heartburn, anesthesia, seizures, vomiting, and weakness. Eating this substance is very dangerous and forbidden.




First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
The hydrazine hydrate should be stored in a tightly-closed container in a cool, dry, well-ventilated area away from incompatible substances and sunlight.






Reviews
There are no reviews yet.