Description
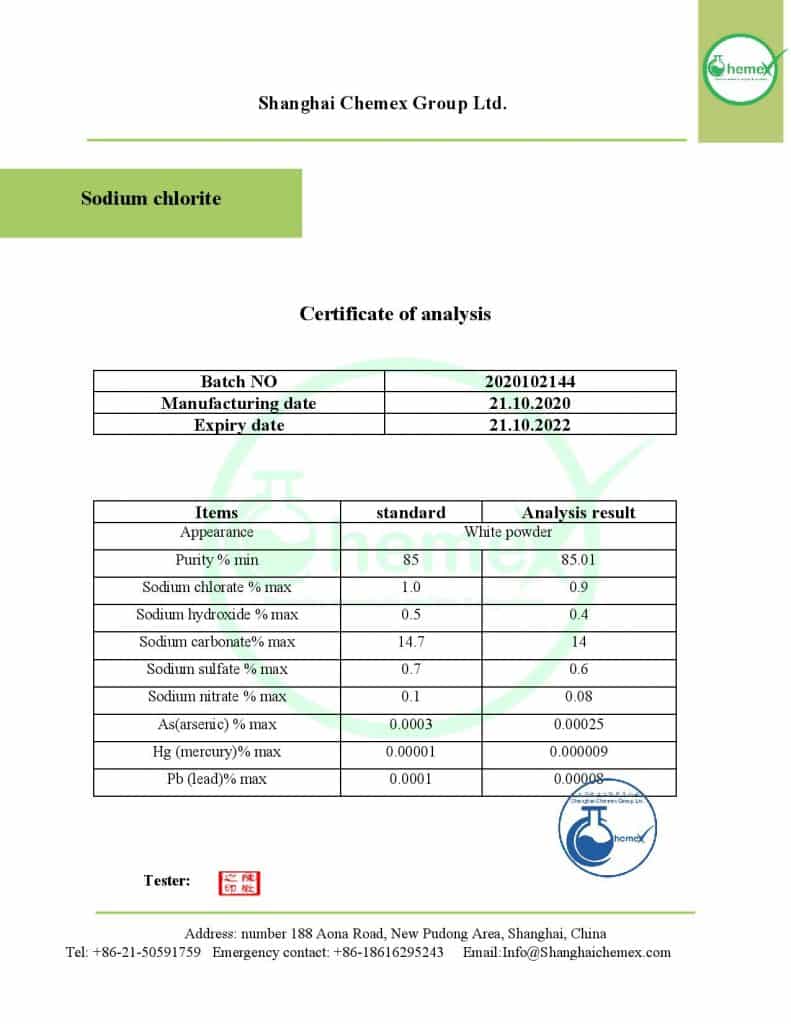
Sodium chlorite is a chemical compound with the formula NaClO2, which is one of the main uses of this substance in the industries related to the production of paper or textiles for bleaching dough or textiles. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
This compound is present in the form of odorless white crystals. It is not flammable alone but accelerates the burning of organic matter and can also cause an explosion when mixed with some flammable materials. On the other hand, it is used as an oxidizing agent in chemical reactions; The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | NaClO2 |
| Molecular Weight(g/mol) | 90.44 |
| Appearance | White solid |
| odor | odorless |
| Density (g/cm3) | 2.468 |
| Melting point (° C) | Decomposes as it melts between 180
-200 |
| Water Solubility | soluble |
| Solubility | Slightly soluble in methanol |
| Synonyms | Chlorous acid, sodium salt
|
| color | White |
| form | crystals or flakes |
| Chemical Structure Depiction | 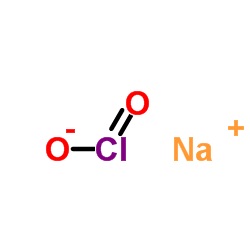 |
Production Process of Sodium Chlorite:
- In general, ClO2, sodium hydroxide, and hydrogen peroxide are the raw materials used in the production of this product. Chlorine dioxide is used as a source of chlorine which is later converted to sodium chlorite.
- Sodium chlorite is obtained indirectly from sodium chlorate. First, sodium chlorate is reduced to chlorine dioxide in a strongly acidic solution using reducing agents such as sodium sulfite, sulfur dioxide, or hydrochloric acid. This substance is then absorbed in an aqueous sodium hydroxide solution, where another reducing agent converts it to sodium chlorite. Hydrogen peroxide can even be used as a reducing agent, producing oxygen gas as a by-product instead of other inorganic salts or substances that can contaminate the product.
Sodium Chlorite Uses:
- Sodium chlorite is used for a variety of applications. as mentioned above sodium chlorite is used for water treatment and also chemical purification.
- It is used as a bleach in the textile industry for a variety of fibers from cotton to synthetic fibers such as nylon.

- In addition, it is used as a bleaching agent in the paper and electronics industries.
- Removal of trihalomethanes from drinking water.
- In addition, sodium chlorite has various industrial uses such as controlling microbial contamination in industrial cooling systems and towers. Because this product is a strong oxidizer, it is commonly used in gas chimney scrubbers.
- Food processing companies use it to wash fruits and vegetables because it is also a strong fungicide. It is also used to wash meat and poultry.
- These compounds are also used for detergents and toothpaste.
- In addition to the industrial applications of this substance, the pharmaceutical grade of this substance is used in the treatment of diseases such as malaria, hepatitis, AIDS, osteoarthritis, colds, etc.
Buy Sodium Chlorite:
Shanghai Chemex is currently one of the suppliers of chemical products in China, which sells sodium chlorite industrial-grade with the best price and quality. Contact our experts to buy the product and place an order.
Safety Information:
- It is a strong oxidant that may cause a fire or explosion.
- Toxic if swallowed.
- Fatal in contact with skin. Causes severe skin burns and eye damage.
- May cause damage to organs through prolonged or repeated exposure.
- Very toxic to aquatic life with long-lasting effects.
- However, using this substance in high doses or for long periods of time can lead to complications such as diarrhea, nausea, insomnia, fatigue, increased saliva, hypotension, etc. In addition, this substance can lead to problems more serious such as nosebleeds, bronchitis, skin irritation, dry throat and cough, and shortness of breath.
- This substance is used in high concentrations in the production of bleach and disinfectants. Exposure to the substance can lead to eye damage, acute respiratory problems, and chemical burns.





First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Don’t induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
The material should be stored in a tightly-closed container in a cool, dry, well-ventilated area.






LIDA –
Is sodium chlorite safe in mouthwash?
china chemicals –
Chlorine dioxide and sodium chlorite are highly reactive disinfectants used to treat public water systems. They are also low-concentration ingredients in some mouthwash products. A sip is unlikely to cause anything beyond mild irritation, nausea, and short-term vomiting.