Description
Tetrapotassium pyrophosphate (TKPP) is a white crystalline powder with hygroscopic properties. It has wide applications in the food industry as an emulsifier and chelation agent. Besides, many bakeries use this substance to improve the taste of the cakes and muffins. It helps maintain the pH levels of various products, so it is a popular ingredient in many industries. Other industries, such as cosmetics and paint, also use it as a main ingredient. In this article, we will explain tetra potassium pyrophosphate properties and applications.
Physical and Chemical Properties of TKPP:
The chemical formula of this compound and its chemical structure is such that it can be easily dissolved in water, Tetra potassium pyrophosphate is not soluble in ethanol and alcohols in general, this compound is also used as an emulsifier, decomposing agent, or tempering in the soap production process. The appearance of this chemical is in the form of granules or white powders that should be avoided as much as possible in direct contact with the skin like Glycine.
This chemical substance is soluble in water but can be mixed with alcohol. The solubility of this chemical substance in water is 187 g at room temperature. This solubility in water makes it a perfect choice to be used as an emulsifier in the food industry.
The Chemical Structure of TKPP is as follows:
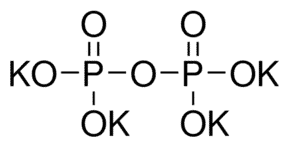
Synthesis and production of TKPP:
The chemical composition of tetra potassium pyrophosphate (TKPP) is produced by the reaction between phosphoric acid and sodium carbonate in a furnace at a very high temperature. These chemical reactions are disodium phosphate, which, if heated to 450 ° C, produces tetra potassium pyrophosphate (TKPP). It can also be produced by the process of molecular dehydration of alkaline sodium phosphate at 500 ° C.
TKPP Uses:
In general, the main use of Tetra potassium pyrophosphate is in the food industry. Tetra potassium pyrophosphate is an emulsifier and buffer in the food industry and to create an almost neutral environment, it is added to food products such as chicken, canned tuna, soy-based foods, animal foods, and so on. Improving the taste of meat foods will be provided with this mineral solid.

Tetra potassium pyrophosphate is used in detergent formulations to improve the quality. The solution of this substance can remove stains and contaminants from clothes, but it should be noted that the presence of this compound in water due to the content of phosphate in its molecular structure, can increase the growth of algae in the water environment; For this reason, the widespread use of this mineral solid as a household detergent is not highly recommended.
This chemical substance is widely used in cosmetic products for controlling the pH level because it is a great buffering agent.
Safety Information:
- People dealing with either of these chemicals must observe all safety precautions for handling corrosive substances. Workers should wear rubber gloves, goggles, and safety masks when working with this acid
- If tetra-potassium pyrophosphate solution comes in contact with the skin of the hand, it can cause severe burning and inflammation.
- TKPP can cause serious damage to the eyes. It is highly recommended that if a person is injured by this substance, they be taken to a medical center immediately.
Packing and Storage:
Packages containing this mineral chemical should be stored in a cool, dry, well-ventilated environment. This material should be stored away from sources of heat generation or oxidizing chemical compounds.






Reviews
There are no reviews yet.