Description
Diethyl hydroxylamine, also known as DEHA, is an organic chemical compound with the chemical formula (C2H5) 2NOH. This substance is considered a colorless liquid and solution and is used as a scrub or oxygen scavenger in the water treatment industry to remove oxygen. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Diethyl hydroxylamine is a colorless, transparent chemical and a derivative of hydroxylamine. It dissolves in water very easily. Chemically Diethyl hydroxylamine is stable and environmentally friendly is non-toxic and its use is not harmful to the environment. It is a reducing agent chemically, so it is oxidized. This substance converts ferric ions to ferrous ions and decomposes in heat and in the presence of water.
The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | C4H11NO |
| Molecular Weight (g/mol) | 89.138 |
| Appearance | colorless liquid |
| odor | Ammoniacal |
| Density (g/cm3 at 20 °C) | 0.8669 |
| Melting point (° C) | −26 to −25 |
| Boiling point (° C) | 127.6 |
| Solubility in water | Miscible |
| Vapor pressure (mm Hg at 25 °C) | 3.36 |
| Flash Point (°F) | 113 |
| Other names | N, N-Diethylhydroxylamine, N-Ethyl-N-hydroxyethanamine |
| Chemical Structure Depiction | 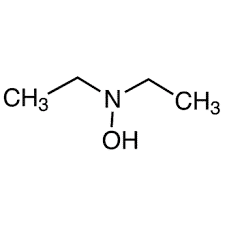 |
The Production Process of Diethyl Hydroxylamine:
The compound can be produced by the process of esterification of adipic acid and ethyl hexanol without the use of a solvent.
The DEHA production method is performed by the reaction of triethylamine and peroxide followed by purification and distillation.
This material is produced in two grades of dry and 85% and both types act as oxygen scrub and radicals.
DEHA Reaction with Oxygen:
From a stoichiometric point of view, 1.2 mg / l DEHA is required to react with 1 mg / l oxygen, but for operational purposes, 3 mg / l DEHA per 1 mg / l oxygen is recommended. Diethyl hydroxylamine oxidation is a complex process that involves various reactions that depend on the temperature, pH, and concentration of DEHA and oxygen.
4(C2H5)2NOH + 9O2 → 8CH3COOH + 2N2 + 6H2O
Diethyl Hydroxylamine Uses:
This material has various applications in the industry. And due to the variety of this substance such as low consumption doses, safety, speed of proper operation, and economical economy, the amount of consumption of this substance and market demand for it increases.
The main applications of diethyl hydroxylamine are:
- Functions as a free radical scavenger in polymerization;
- This compound is used as an oxygen scavenger for medium and high- pressure boilers;
- Polymerization inhibitor in the processing of different polymers;
- Electronic chemicals;
- Anti-corrosion;
- Color stabilizer (photography).
Oxygen Scavenger:
Specifically, the oxygen in the water used in power plants, refineries, and equipment of water treatment systems is known as a destructive factor that can cause undesirable phenomena in the long-term run. Hydrazine is often used in power plants to eliminate the risk of corrosion caused by water-soluble oxygen. However, this substance has adverse effects and high toxicity and its effect on increasing the rate of carcinogenesis has been proven. And in order to eliminate the dangers of this substance, much research has been done to replace other substances with hydrazine. Carbohydrazide and diethyl hydroxylamine (DEHA) are among the substances used as substitutes in these industries.

Safety Information:
- May cause eye and skin irritation.
- Avoid swallowing DEHA seriously as it can have adverse effects and inflammation of the gastrointestinal tract.
- May cause respiratory tract irritation. Avoid inhaling vapors from this chemical, irritation of the respiratory system, lungs and headache and dizziness are the effects of inhaling vapors.
- In general, for fish and aquatics, this inorganic compound is considered toxic and is harmful to aquatics’ health.
The use of this substance should be reduced as much as possible and it should be used as required and in standard amounts, Excessive consumption can negatively affect its performance and adverse effects on the environment in the long run.


First-aid measures:
Skin Contact: Immediately flush skin with water.
Inhalation: move the person to the fresh air.
Eye Contact: Rinse eyes with water for at least 15-20 minutes.
Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center
When working with this inorganic material, pay attention to all safety and health points, use safety glasses, gloves, and special clothes.
Packing and Storage:
Store in a cool, dry, well-ventilated area away from incompatible substances, heat, sparks, and flame.






Reviews
There are no reviews yet.