Description
Cyclohexylamine is an organic compound from the group of aliphatic amines and is known as aminocyclohexane and cyclohexane amine. Cyclohexylamine, like other amines, has a weak base compared to strong bases such as sodium hydroxide, but a stronger base than its aromatic analogs. This compound is a useful mediator in the production of many other organic compounds such as cyclamate.

It was introduced in 1893 but was not used economically in the United States until 1936. But today it is one of the most highly produced chemicals in the chemical industry.
And after the United States, Asia, especially China, accounted for about 65% of total global production capacity.
Physical and Chemical Properties:
Cyclohexylamine with the chemical formula C3H13N is a colorless liquid that, however, may sometimes appear colored due to the presence of contaminants. It smells like fish and can be mixed with water and other organic solvents such as alcohols, ethers, ketones, and aliphatic and aromatic esters.
The most important physical and chemical properties of this compound can be summarized in the following table:
| Chemical formula | C6H13N |
| Molecular Weight(g/mol) | 99.17 |
| Appearance | liquid |
| odor | strong, fishy, amine odor |
| Density (g/mL at 25 °C) | 0.8647 |
| Melting point (° C) | −17.7 |
| Boiling point (° C) | 134.5 |
| Water Solubility | Miscible |
| Solubility | very soluble in ethanol, oil
miscible in ethers, acetone, esters, alcohol, ketones |
| Vapor pressure (mmHg at 20° C) | 11 |
| Flash Point (°F) | 88 |
| Other names | Aminocyclohexane, Aminohexahydrobenzene, Hexahydroaniline, Hexahydrobenzenamine |
| color | clear to yellowish |
| form | liquid |
| Chemical Structure Depiction | 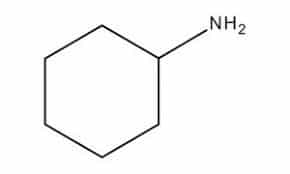 |
Synthesis of Cyclohexylamine:
This substance is produced by two methods, one of which is the reaction of aniline hydrogenation in the presence of cobalt or nickel as a catalyst according to the following reaction:
C6H5NH2 + 3 H2 → C6H11NH2
The second method for the synthesis of this substance is the use of an ammonia alkylation reaction in the presence of cyclohexanol.
Cyclohexylamine Uses:
Cyclohexylamine is an intermediate compound in the synthesis of many organic compounds. This compound is a precursor to sulfonamide reagents that are used as accelerators. Amines themselves are effective inhibitors of corrosion. It acts alone or as a soap, moisturizing agent as well as cleanser and emulsifier.
Used in the pharmaceutical industry in the production of analgesics
Used in agricultural industries in the production of some herbicides.

In the oil and gas industry, it is used in the synthesis of many organic compounds, corrosion and precipitate inhibitors, neutralization of raw materials, water purifiers for boilers, and additives in petroleum products.
It is used in the cosmetics industry as a raw material for the production of some perfumes.
It is used as a solvent in the printing and dyeing industries.
Water treatment and wastewater treatment
Production of PVC adhesive like Tetrahydrofuran
Safety Information:
This material is flammable due to its low flash point (27° C).
It is inedible, swallowing and inhaling large amounts of this substance can be fatal.
It is rapidly absorbed through the skin, which can cause inflammation and corrosion in the body.
According to the defined standards, this substance is known as a dangerous substance.

the standard amount of cyclohexylamine that is allowed to be exposed by workers is less than 10 ppm (40 mg/m3) through an eight-hour work shift.
Packing and Storage:
Cyclohexylamine should be kept in a tightly-closed container away from open flames, sparks, heat, or hot surfaces as it is highly flammable






Reviews
There are no reviews yet.