Description
Dimethylglyoxime, also known as Diacetyldioxime or the Sodium salt of acetaldoxime, is a chemical compound produced by the reaction of butanone with ethyl nitrite in the presence of a hydrochloric acid catalyst. The main application of dimethylglyoxime is for the detection of metals such as platinum, palladium, and nickel. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.
In the following, we will get more acquainted with this chemical and its properties and main applications.
Chemical and Physical Properties of Dimethylglyoxime:
The chemical formula of this compound is C4H8N2O2 and its appearance in standard conditions and at ambient temperature and pressure is as a colorless crystal or white and odorless powder, which acts as a bidentate ligand due to having two hydrogen atoms in its structure.
The chemical structure of this compound is such that it is difficult to dissolve in water, but it can be well dissolved in methanol, ether, pyridine, acetone as well as sodium hydroxide solution. It is used in laboratories and research centers to detect enzymes.
The following table deals with the physical and chemical properties of this material:
| Name | Dimethylglyoxime |
|
Chemical formula |
C4H8N2O2 |
| Molecular weight (g/mol) | 116.12 |
| Appearance |
White Powder |
| Density (g/cm³) | 1.37 |
| Melting point (°C) | 240 |
| Boiling point (°C) | Indefinite |
| Solubility in water | Very low |
|
Chemical Structure Depiction |
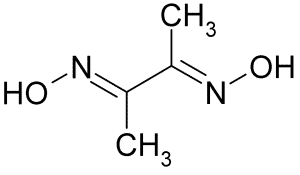 |
What type of ligand is Dimethylglyoxime?
In the previous section, we mentioned that dimethylglyoxime is known as the exclusive nickel ligand, but what does ligand mean?
A ligand is a molecule that can bind to a central metal atom. The chemical structure of dimethylglyoxime is such that it binds well to the metal nickel or palladium. As they gain weight, they cause deposition and separation. Ligands are electron donors, and the strength of the power of a ligand bond is directly related to its active site. Dimethylglyoxime can be classified as a bilateral ligand and its chemical structure indicates that it has donor atoms on both sides that can form strong ionic and metal complexes.
The Production Method of Dimethylglyoxime:
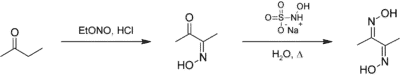
To prepare this compound, butanone is first combined with ethyl nitrite to produce bicyclic monoxime. Sodium hydroxylamine monosulfonate is then used to add another oxime group to the final product. To perform and advance this reaction, an acid catalyst is needed in the first stage, and heat is needed in the second stage. You can see the reaction of preparing this compound in the figure below:
Dimethylglyoxime Uses?
- Used as a precipitate for palladium and nickel. Palladium precipitate in this reaction turns yellow when diluted with hydrochloric acid.
- DMG helps diagnose dermatitis or similar skin diseases. Nickel released from watches detects jewelry, etc. that is in direct contact with our skin.
- Widely used in decomposition chemistry as a reagent, precipitator as well as photometric reagent of metal ions such as platinum, palladium, and nickel.
- In the hydrometallurgical process, cathodic waste is used to wash lithium, cobalt, and nickel from batteries.

Buy and Sell Dimethylglyoxime:
You can contact our experts in Shanghai Chemex to buy this product with high quality and reasonable price.
Safety Information:
Pay attention to all the safety and health points when working with this material, and it is better to avoid high heat or smoking in the warehouse area. DMG can have severe health effects if exposed to large amounts.
- It can cause damage to the eyes and skin.
- Eating it stimulates the gastrointestinal tract and damages it.
- Inhalation can irritate the respiratory tract and damage the mucous membranes.
- In addition, due to its flexible structure, it can harm the environment. When decomposed, it produces toxic gases that lead to combustion.
Packing and Storage:
Store in a tightly-closed container in a cool, dry, well-ventilated area away from incompatible substances.






Reviews
There are no reviews yet.