Description
Zinc chloride is a chemical and salt compound that exists in both monohydrate and anhydrous forms. So far, 9 crystal structures have been identified from this compound. In addition to the solid-state, it is also available as 50% and 70% solutions. Due to its high solubility, it is used in many different industries, including the paper industry, textile, disinfectants, and hygiene products. Shanghai Chemex is one of the most reputable manufacturers of this chemical in the world.

Physical and Chemical Properties:
Zinc chloride with the molecular formula ZnCl2 appears as a liquid, powder, and white granules that decompose due to heat and emit zinc oxide and hydrogen chloride gases. Its vapors have a pungent odor and are corrosive to metals. This compound is a moisture absorber and must be protected against moisture and even water vapor in the air; In the table below some of the chemical and physical properties of this product are mentioned:
| Chemical formula | ZnCl2 |
| Molecular Weight (g/mol) | 136.315 |
| Appearance | solid |
| Odor | odorless |
| Density (g/cm3) | 2.907 |
| Melting point (° C) | 290 |
| Boiling point (° C) | 732 |
| Solubility | soluble in water, ethanol, glycerol, and acetone |
| Color | White |
| Form | granules |
|
Chemical Structure Depiction |
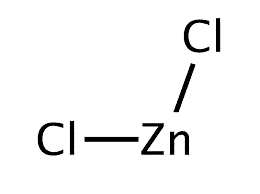 |
The Production Process of Zinc Chloride:
The reaction of zinc and hydrochloric acid is used to produce anhydrous zinc chloride. During this reaction, ZnCl2 and hydrogen gas are produced. The chemical equation of the reaction is as follows:
Zn(s) + 2 HCl → ZnCl2 + H2(g)
Its hydrated form and an aqueous solution of zinc chloride can be prepared by reacting zinc metal and various salts of zinc (such as zinc carbonate, zinc oxide, and zinc sulfide) with hydrochloric acid.
ZnS + 2 HCl + 4 H2O → ZnCl2(H2O)4 + H2S
Commercial samples of zinc chloride contain water and products from the hydrolysis of impurities. Some of these impurities can be obtained by recrystallization from hot dioxane.
Zinc Chloride Uses:
Zinc chloride is used in various industries. Important applications of this material can be mentioned as follows:
- As a Lewis acid used in laboratory syntheses
- In welding as welding wire
- As an electrolyte in solar cells
- To separate oil from water and reduce the stability of oil in water
- It is one of the important elements in plant growth
- In the paint industry as a color stabilizer
- One of the alternative treatments for skin cancer
- In the production and protection of wood and adhesives
- Production of ion-exchange resin
Zinc Chloride in Metallurgy and Welding:
Due to the ability of zinc chloride to attack metal oxides, this material is used as a flux or flux in metallurgy and soldering. In addition, this material is also used in galvanized welds and coatings.
The Role of Zinc Chloride in Wood and Adhesive:
One of the uses of chloride is its use in the adhesive and wood industry. This material is useful for the production and preservation of adhesive and is also used in wood impregnation. Together with sodium dichromate, zinc chloride helps preserve the wood.

Buy Zinc Chloride:
Zinc chloride is one of the compounds that is always considered due to its importance and many applications in various industries. Contact our experts in Shanghai Chemex to place an order and find out the price of this product.
Safety Information:
When consumed in excess, it can cause poisoning, severe stomach irritation, nausea, vomiting, and diarrhea. It is also very dangerous for the environment and has high durability.


First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
Keep in a tightly closed container. Store in a cool, dry, ventilated area away from strong bases, and strong oxidants.






Reviews
There are no reviews yet.