Description
Potassium Acetate is one of the most important salts of acetic acid with the chemical formula C2H3KO2. This potassium acetic acid salt is found in most fruits and is produced by bacterial fermentation. This inorganic compound acts as an antioxidant and is used as a food additive. This chemical is used as a substitute for chloride salts such as sodium chloride, calcium chloride, and magnesium chloride. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and chemical properties:
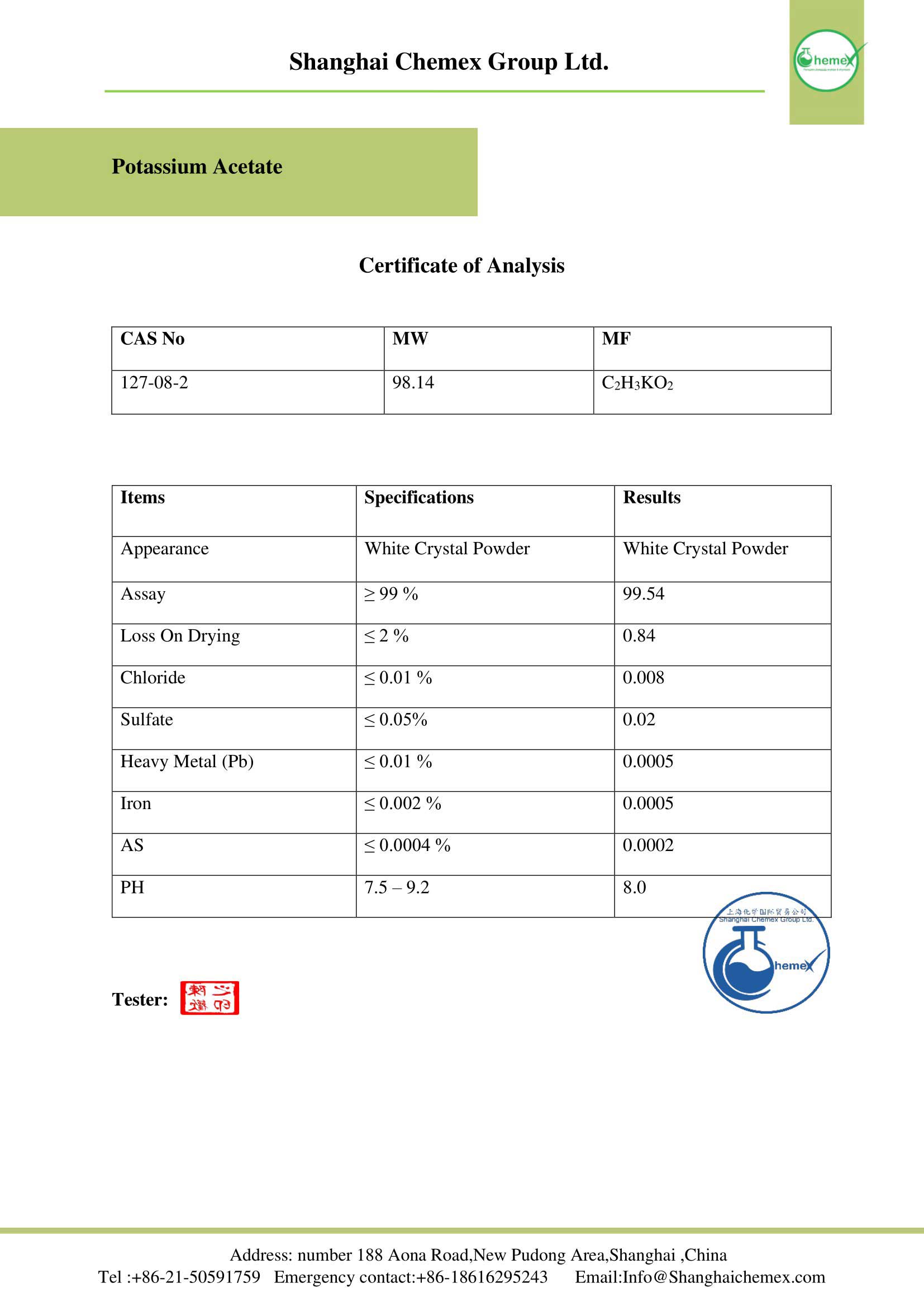
Potassium Acetate is a chemical compound with a molecular mass of 98.14 g / mol. The appearance of this compound is a white powder. This neutral salt is soluble in water and has an acidic odor. This salt is one of the organometallic compounds. In the table below some of the chemical and physical properties of this product are mentioned:
| Chemical formula | C2H3KO2 |
| Molecular Weight (g/mol) | 98.142 |
| Appearance | The white deliquescent crystalline powder |
| Density(g/cm3 at 25 °C) | 1.57 |
| Melting Point(° C) | 292 |
| Boiling point | Decomposes |
| Solubility in water(g/100 mL at 25 °C) | 268.6 |
| Solubility | Soluble in alcohol, liquid ammonia
Insoluble in ether, acetone |
| PH | 7,5-9,0 |
| Acidity (pKa) | 4.76 |
| color | white |
| form | crystalline powder |
| Crystal structure | Monoclinic |
| Chemical Structure Depiction | 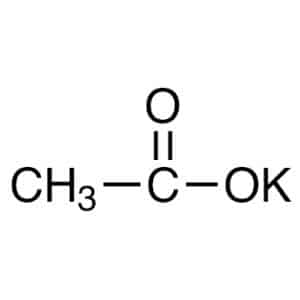 |
Structure and Formula of Potassium Acetate:
The chemical formula of potassium acetate is CH₃COOK. As is clear from the formula, potassium acetate is composed of an anion acetate and a potassium cation bonded with ionic bonds.
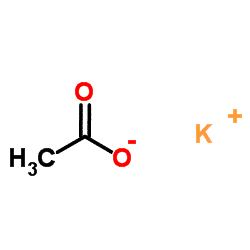
Potassium Acetate Solubility:
This chemical has good solubility in water. This property increases with increasing temperature. This increase is such that at 20 and 62 ° C the solubility in water is equivalent to 253 and 492 g / 100 ml, respectively. This statistic indicates that this substance is associated with a 40 ° C increase in temperature, doubling the solubility in water.
Production of Potassium Acetate:
- This salt is found in most fruits and is produced by bacterial fermentation.
- This compound is obtained by the reaction of potassium hydroxide or potassium carbonate with acetic acid. This reaction is known as an acid neutralization reaction.
CH3COOH + KOH → CH3COOK + H2O
- Production of this substance in industry and commercial grade is also produced from bacterial fermentation of sugar, alcohol, or by chemical synthesis of acetaldehyde.
Potassium Acetate Uses:
- Potassium acetate is used as an antifreeze to prevent its formation as an alternative to chloride salts such as calcium chloride or magnesium chloride on roads and airport runways.
- This compound is used in antibiotics, Penicillin, insulin, and dialysis solutions.
- This substance in chemical fertilizers is a source of potassium
- As a food additive (preservative and acidity regulator)
- It is also used in textiles, fire extinguishers, biochemistry
- a catalyst in the production of polyurethane foam and carbon black
- Preservation of mummified tissues
- Making glass
- Softening agent in paper and textile
- Oxidizing
Defrosting industries:
This chemical is used in the defrost industry as an antifreeze or a desiccant to remove ice and prevent it from forming. Potassium acetate is used as a substitute for chloride salts such as sodium chloride, calcium chloride, and magnesium chloride. The advantage of this material compared to other materials is its safety in its effects on soils and surfaces. It has very little corrosion which makes it used on roads and airport runways, which is expensive but cheaper than other chlorides.

Medical industry:
This chemical is used in the treatment of diabetic ketoacidosis due to its unique properties in alternative protocols. Its effective properties include the ability to decompose into potassium bicarbonate, as well as helping to neutralize the acidic state.
This inorganic compound is also a key ingredient in the body’s natural functions such as:
- Maintain blood pressure
- Kidney function
- Synthesis of nucleic acids
- Produces the energy needed by the body
- Nerve conduction of nerve cells
This chemical is used as a strong antihypertensive in the body. Therefore, this substance is used in dietary and sports supplements.
The drugs used in this substance are:
- Insulins
- Penicillin
- Antibiotics
- Dialysis solutions

Safety information:
- Harmful if swallowed
- Causes skin irritation
- Causes serious eye irritation
- May cause respiratory irritation

First-aid measures:
Skin Contact: Immediately flush skin with water.
Inhalation: move the person to the fresh air.
Eye Contact: Rinse eyes with water for at least 15-20 minutes.
Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and Storage:
Store in a cool, dry place. Keep the container tightly closed, and away from incompatible materials.






Reviews
There are no reviews yet.