Description
Calcium chloride is an inorganic compound that is marketed in 36% soluble, 75%- 78% crystal, or 94%- 97% granules. This material has a wide range of applications in a variety of industries, used for road defrosting, dust control, dehumidification, and setting the reduction time of concrete, in the oil industry as well as in oil extraction and food processing. The process of producing calcium chloride is basically the reaction between limestone and hydrochloric acid. It can also be produced as a Solvay by-product for soda ash. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.
Physical and Chemical Properties:
Calcium chloride is an ionic compound also known as calcium dichloride. This material is available in both food and industrial grades in the market. It is solid at room temperature, but also available as a solution. One of its interesting features is the release of heat when dissolved in water, which is due to the high enthalpy solubility of this material; The following table identifies the most important physical and chemical properties of this material:
| Chemical formula | CaCl2 |
| Molecular Weight(g/mol) | 110.98 |
| Appearance | solid |
| odor | Odorless |
| Density (g/cm3) | 2.15 |
| Melting point (° C) | 782 |
| Boiling point (° C) | 1935 |
| Chemical Structure Depiction | 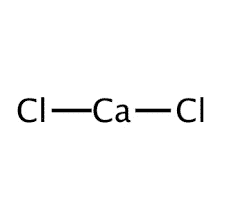 |
Synthesis of Calcium Chloride :
The first method:
CaCl2 can be produced by the reaction between calcium carbonate (limestone) and hydrochloric acid with the following reaction equation.
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
If hydrochloric acid (36%) is used, CaCl2 can be separated with a purity of 40%, and purification of the product is mainly done by adding Ca (OH) 2 as in the natural saltwater process.
A limestone reaction is used to produce calcium chloride in many countries. If the purity of hydrochloric acid and limestone is sufficient, completely pure calcium chloride can be obtained using this process. Hence, this process is suitable for food production. It is also an environmentally friendly method for using hydrochloric acid.
The second method:
The most commonly used method in the United States is to concentrate and purify water or salt deposits in lakes. Existing sediments may contain some magnesium. They use the chemical Ca (OH) 2 to purify it and precipitate magnesium as Mg (OH) 2.
Ca(OH)2 + Mg2+ → Mg(OH)2 + Ca2+
Existing impurities are also removed by sedimentation. During the water evaporation process, NaCl salt is precipitated to increase the concentration of the desired product and pure Calcium dichloride is obtained. The advantages of this method include the cheapness of raw materials and low environmental risks. But the purity of the product obtained during this method is less than in the previous method.
Third method:
Calcium carbonate can be produced as a by-product of the Solvay process.
Calcium Chloride Uses:
Food:
Calcium chloride is available as a preservative in a variety of snacks, beverages, sports drinks, and specialty products. CaCl2 not only prevents food spoilage but also provides the body with essential mineral calcium and enhances the nutritional quality of the product. Due to high concerns about the high sodium content in foods and insufficient calcium intake, many manufacturers add calcium chloride to their products instead of sodium chloride.
Agricultural:
One of the minerals needed for plant growth and cell formation is calcium. To increase the amount of calcium in the soil, calcium chloride is added to the soil in a certain percentage as a solution. This increases the strength of the plant walls.
Oil and Gas Industry:
In the petrochemical industry, this product can be used to remove water from hydrocarbons, because this material has the property of absorbing moisture and can be used to dry materials such as ethane, butane LPG, hydrocarbons and aromatic hydrocarbons, propane, diesel fuels be useful.
Prevent Icy Roads:
This mineral can be used as an antifreeze and freezing point reducer. Simultaneously with reducing the freezing point, calcium water is used to prevent water freezing, especially on road surfaces. like Potassium Acetate

Other Industries:
Other applications of this product include its use in dust control, fluoride removal, ice and snowmelt, animal feed, and…
Buy Calcium Chloride:
For information on how to sell this product and place an order, you can contact our partners in the Shanghai Chemex sales department.
Safety:
It is better to observe all safety items such as the use of gloves and safety glasses and also the use of special clothing when working with this material. In case of direct contact of this material with the skin, wash the contact area immediately with soap and water and avoid direct contact with it. Wear long-sleeved clothing and a helmet.
Transporting and Storage:
The storage of calcium chloride should be equipped with a proper ventilation system so that small particles do not enter the respiratory system. Store this material in a cool and dry place away from heat.



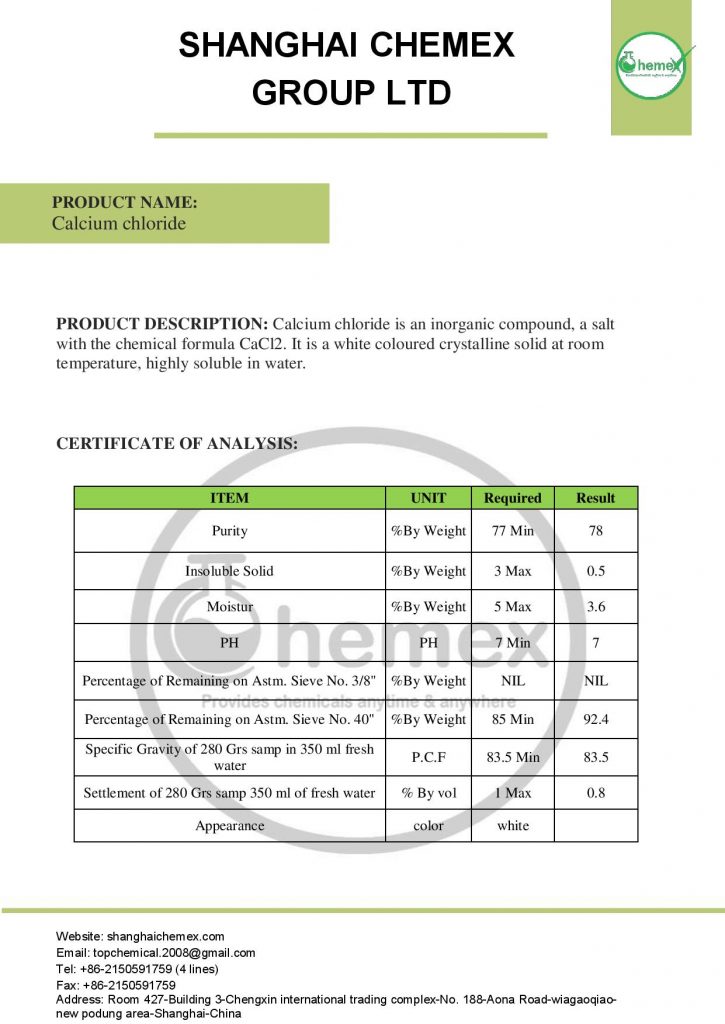 some text here
some text here



Reviews
There are no reviews yet.