Description
Sodium benzoate is the sodium salt of benzoic acid and has the chemical formula C7H5NaO2. This chemical compound is added as a preservative to some beverages, processed and packaged foods, and care products with the goal of extending their life and preventing or delaying microbial, enzymatic, and chemical spoilage. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Sodium benzoate is a white, odorless crystalline compound that has a sweet taste and is soluble in water. Because it is the inactive salt of sodium benzoic acid, it forms benzoic acid when dissolved in water. One way to produce this substance is by reacting sodium hydroxide with benzoic acid; In the table below some of the chemical and physical properties of this product are mentioned:
| Chemical formula | C7H5NaO2 |
|
Molecular Weight (g/mol) |
144.105 |
| Appearance | Solid |
| Odor | odorless |
| Taste | Sweetish, astringent taste |
| Density (g/cm3) | 1.497 |
| pH | about 8 |
|
Melting point (° C) |
410 |
| Boiling point (° C) | 450 to 475 |
| Solubility | soluble in water, liquid ammonia, and pyridine |
| Color | White or Colorless |
| Form | granules or crystalline powder |
| Chemical Structure Depiction | 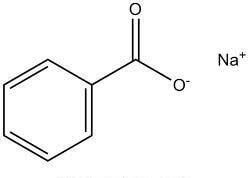 |
Structure of Sodium Benzoate:
The above chemical composition is an organic sodium salt resulting from the replacement of the proton from the carboxy group of benzoic acid by a sodium ion.
Sodium Benzoate Production Process:
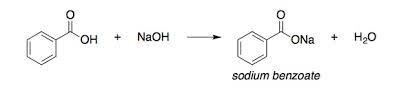
Industrial production of sodium benzoate is generally done from a combination of benzoic acid with NaOH solution. There are two different ways to do this process, and the difference between the two methods is in how benzoic acid is produced. In this process, toluene is oxidized by nitric acid, and eventually, benzoic acid is produced. Another way to produce this acid is to hydrolyze benzo trichloride and then add a mineral acid to it. The most common way to convert benzoic acid to the above product is to add this acid to a solution of sodium hydroxide. The solution of this process contains Sodium Benzoate and water, which can be obtained by evaporation of the desired salt crystals.
Sodium benzoate is not naturally present, but benzoic acid is found in many plants, including cinnamon, tomatoes, berries, plums, and apples.
Sodium Benzoate Uses:
Food:
Sodium benzoate is a preservative also known as E211. Used in acidic foods such as salad dressings, carbonated beverages, jams and juices, pickles, condiments, and frozen yogurt additives. Prevents the growth of harmful bacteria, mold, and other germs in food, thus preventing spoilage.

Pharmacy:
Recent research suggests that sodium benzoate may be useful as a treatment for schizophrenia. It is also used in combination with caffeine to treat headaches and shortness of breath caused by overdose.
Cosmetics:
Commonly used as a preservative in cosmetics such as hair products, baby wipes, toothpaste, and mouthwash. The reason for using preservatives in cosmetic products is that the growth of microorganisms in these products can cause unpleasant odors and colors.
Other uses include:
Industrial uses of sodium benzoate can be used in car engine coolers as an anti-corrosion agent. In addition, it can be used to stabilize the photographic process or improve the consistency of some plastics.
Buy Sodium Benzoate:
Due to the fact that sodium benzoate is a combination with a wide range of applications and is often used as a preservative in the food industry, the quality and originality of the product are of great importance for the food grade. Shanghai Chemex is one of the reputable suppliers in the field of selling various industrial and food chemicals, which has made the quality of products a priority. To know the price of sodium benzoate and product features and receive its analysis, you can contact our sales experts.
Safety Information of Sodium Benzoate:
A major concern about the use of sodium benzoate is its ability to convert to benzene, which is known to be a carcinogen. Benzene can be found in soft drinks and other beverages that contain sodium benzoate and vitamin C (ascorbic acid). It is important to note that diet drinks are more prone to the formation of benzene, as the sugar in regular sodium and fruit drinks can reduce its formation. Your body does not store sodium benzoate. Instead, you metabolize and excrete it in your urine within 24 hours, which helps keep it safe. The FDA allows the concentration of 0.1% of this product by weight in foods and beverages and, if used, should be included in the list of ingredients. Other factors, such as exposure to heat and light, as well as longer storage times, can increase benzene levels.

Packing and Storage:
Store in a tightly-closed container in a cool, dry, well-ventilated place away from incompatible materials.






Reviews
There are no reviews yet.