Description
Sodium diacetate is the sodium salt of acetic acid with the chemical formula C4H7NAO4. This compound is composed of sodium acetate and acetic acid and has a wide range of uses as an antibacterial. The use of this antibacterial in packaged foods and fast foods increases their nutritional value and increases the shelf life of this salt. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Sodium diacetate is the same sodium acetate with a slight taste of acetic acid that is used as a sour substance and pH regulator. This substance is prepared from the combination of 58% sodium acetate (dehydrated) and 42% glacial acetic acid and it is also called dry acetic acid. In the chemical structure of this preservative, carbon, oxygen, and hydrogen atoms have been used and this white crystal has a high solubility in water, it is compatible with ethanol, methanol, and hydrazine and it forms a uniform solution its other names are sodium ethanoate and sodium acetic acid salt; The physical and chemical properties of this antibacterial can be summarized in the following table:
| Name | sodium diacetate |
| Chemical formula | C4H7NaO4 |
| Molecular weight (g/mol) | 142.03 |
| Appearance | White powder |
| odor | Acetic acid odor |
| Density (g/cm3) | 1.5 |
| Melting point (°C) | 300 |
| Solubility | soluble in water, alcohol, and insoluble in ether |
| Chemical Structure Depiction | 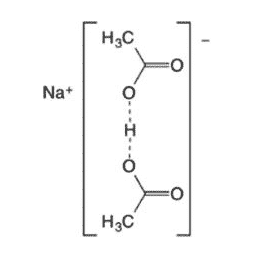 |
Synthesis and Production of Sodium Diacetate:
The process of producing sodium diacetate is a synthetic reaction between acetic acid and sodium carbonate. To perform this reaction, a high-speed agitator at 100 to 210 rpm and a pressure reactor at 40 to 50 ° C are required. In the overall structure of this compound, 39 to 41% of CH3COOH and 58 to 60% of CH3COONa are present. It can also be prepared directly from the neutralization of acetic acid using sodium hydroxide. The reaction equation is as follows:
2 CH3CO2H + NaOH → Na+[(CH3CO2)2H]− + H2O
Sodium Diacetate Uses:
In general, this compound can be used in food, animal feed, cosmetics, and pharmaceutical industries due to its antimicrobial properties, vinegar taste, and pH adjustment, which are described separately below:
Food:
In general, the food grade of these compounds is used in bakery products, meat, and salt products. In addition, the solid form of this material makes it easier to use and transport than liquid acetic acid.

Acidity Regulation:
This compound acts as a buffering agent to regulate food acidity and can be easily used, either dry or liquid. like Sodium acetate
Animal Feed:
It can be used as a preservative in animal feed, poultry, pigs, and other animals.
Cosmetics:
According to the European Commission database, this substance can act as a buffer and cover substances in cosmetics and personal care products.
Pharmaceutical:
Sodium diacetate plays an important role in regulating the pH of the dialysis solution and is only stored dry in clinics because it is easier to maintain than in the liquid form.
Buy Sodium Diacetate:
Shanghai Chemex, active in the field of chemical sales, offers sodium diacetate to customers at the best quality and the most appropriate price. To buy this product, contact our experts.
Safety:
One question that may be on the minds of some people about sodium acetate is whether the use of this antibacterial in the food industry is dangerous for human health or whether it can be carcinogenic. Reports of consumption of food products in which this salt has been used have not seen any bad effects or carcinogenic effects. It is a safe compound approved by the US Food and Drug Administration (FDA), the European Food Safety Authority (EFSA), and the FAO / WHO Joint Committee. Also, its dangers have not been reported so far and may cause allergic reactions in some people.

Packing and Storage:
Store and use in a dry, cool, well-ventilated place away from heat, sparks, open flames, or any other source of ignition.






Reviews
There are no reviews yet.