Description
Sodium acetate is a highly functional chemical compound known as one of the sodium salts and is available in two types anhydrous and trihydrate in the market. This salt is used as one of the raw materials used in the food production industry as a preservative, pH regulator, and emulsifier. In addition, this substance is also used in industry, laboratories, and the production of cosmetic products. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Sodium acetate is a salt with the chemical formula C2H3O2Na, also known as sodium ethanoate. This chemical appears as a white granular powder and monoclinic crystals. Its nature is moisture absorbing and dissolves easily in water. This compound is usually odorless but gives off an odor of vinegar or acetic acid when heated to the point of decomposition; In the table below some of the chemical and physical properties of this product are mentioned:
| Chemical formula | CH3COONa |
| Molecular Weight (g/mol) | 82.03 |
| Appearance | Wet Solid |
| odor | Vinegar odor |
| PH | 8,0-9,5 |
| Density (g/cm3) | 1.528 |
| Melting point (° C) | 324.0 |
| Boiling point (° C) | 881.4 |
| Solubility | Soluble in water, alcohol, hydrazine |
| Color | White |
| Form | powder |
| Chemical Structure Depiction | 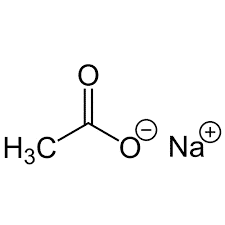 |
The Production Process of Sodium Acetate:
The simplest and fastest way to produce sodium acetate is to neutralize acetic acid with sodium carbonate. After drying and removing water from it, crystalline solids of sodium acetate appear, the reaction of which is as follows:
NaHCO3 + CH3COOH —> CH3COONa + CO2 + H2O
Another method of producing the above product is the reaction of acetic acid with alkalis such as sodium hydroxide.
Sodium Acetate Uses:
Sodium acetate is a very useful compound in various industries, including the textile industry, food industry, construction materials industry, and…. Other uses for this compound include in the production of photographic films, in the production of cosmetics, in the laboratory, in the production of pigments, and in the oil industry. In the following, we will explain each of these cases.
Lab:
This compound is a widely used substance in molecular biology and biochemistry laboratories, one of the applications of which is to remove DNA from cells.
Building Materials:
One of the applications of sodium acetate in the construction materials industry is to increase the life of the concrete. This compound is used in concrete to reduce water damage as insulation for concrete. This compound is more economical and also less environmentally friendly than epoxy previously used to make concrete water-resistant.
Cosmetics:
This compound is used in the production of cosmetics with a high PHP range as a preservative. It is also used in the production of hair dyes and shampoos due to its buffering properties.
Textile:
The use of sodium acetate in the textile industry is to neutralize sulfuric acid residues. This compound is also used to prevent static electricity from accumulating in the cotton garment production process.
Food:
Another use of sodium acetate in the food industry is as a seasoning and flavoring. It is also added to food to prevent the growth of bacteria and has a preservative role.

Buffer solutions:
A solution of sodium acetate (acetic acid salt) and acetic acid can act as a buffer to maintain a relatively constant pH level. This solution has many biochemical applications, especially when the reactions are pH-dependent and need to be in the low acid range (4 to 6). like Trisodium citrate
Buy sodium acetate:
Shanghai Chemex offers sodium acetate products in two types juicy and dry and in two grades of industrial and food with the best quality and price. To register the order and know the price of the product, please contact the experts in our sales department.
Side Effect:
- Flammability: It has the ability to ignite and fire.
- Eye contact: This substance can cause red eyes.
- Skin contact: Causes inflammation and redness of the skin.
- Contact with the respiratory tract: Breathing powders and gases can cause coughing, sneezing, and sore throat.
First-aid:
- When in contact with skin, wash immediately with soap and water.
- Use water spray and powder to extinguish the fire during a fire.
- After inhaling these substances, take the person to fresh air to rest and breathe in that space.
- When the material comes into contact with the eyes, immediately rinse the eyes with plenty of water for 15 minutes and then perform therapeutic measures.
Packing and storage:
Sodium acetate or sodium ethanoate is a flammable substance and can ignite if exposed to heat. Therefore, this chemical should be stored away from any heat source and in a cool, dry, and well-ventilated place.






Reviews
There are no reviews yet.