Description
Sodium sulfide, also known as disodium sulfide, is a white solid and is soluble in water, which can form alkaline solutions. Sodium sulfide and its hydrates exposed to air can release hydrogen sulfide (H2S) gas, which has a very pungent odor (similar to that of rotten eggs). This compound, in reaction with hydrogen peroxide, produces sodium sulfate. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Sodium sulfide is a chemical compound with the formula Na2S and its hydrated form with the formula Na2S.9H2O, both of which are water-soluble salts and produce strong, colorless alkaline solutions. Although this compound is a yellow solid, its solution is colorless. Sodium sulfide is produced in crystalline, flaky, and broken forms. Industrial sodium sulfide is usually present in brick yellow and red due to the presence of polysulfides; In the table below some of the chemical and physical properties of this product are mentioned:
| Chemical formula | Na2S |
| Molecular Weight(g/mol) | 78.0452(anhydrous)
240.18 (nonahydrate) |
| Appearance | Solid |
| Density(g/cm3) | 1.856 (anhydrous)
1.58 (pentahydrate) 1.43 (nonahydrate) |
| odor | Rotten eggs |
| Melting point(° C) | 1,176 (anhydrous)
100(pentahydrate) 50(nonahydrate) |
| Boiling point(° C) | Very high |
| Solubility in water | Soluble |
| Solubility | insoluble in ether
slightly soluble in alcohol |
| color | Yellow or brick-red |
| form | lumps or flakes or deliquescent crystals |
| Chemical Structure Depiction | 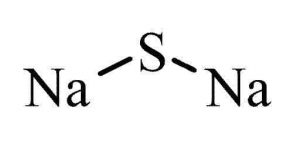 |
Types of Sodium Sulfide:
Crystallized:
In the crystallized state, each molecule of sodium sulfide has crystallized between 7 and 9 molecules of water, and its purity is about 29 to 30%.
Flakes:
At the end of the production process, sodium sulfide forms flakes during operations such as cutting, in which case sodium sulfide has a purity of about 60%.
Broken:
This compound is obtained as a lump by breaking large pieces after drying and has a purity of 55 to 60%.
Sodium Sulfide Production Process:
There are generally three methods for producing sodium sulfide:
- Reduction of sodium sulfate with coal
Na2SO4 + 2 C → Na2S + 2 CO2
- The reaction of hydrogen sulfide and sodium hydroxide (caustic soda)
- In the laboratory, this salt can be produced by reducing sulfur with sodium in the presence of aqueous ammonia.
2 Na + S → Na2S
Production of Sodium Sulfide by Reduction of Sodium Sulfate with Coal:
In industry, sodium sulfide is obtained from the reduction of sodium sulfate with coal. These raw materials are found in abundance in nature. This reaction takes place inside a furnace at a temperature of 1000 ° C. To this reaction is added 0.5% sodium carbonate. It acts both as a catalyst and as a neutralizing agent for any free acid. The product obtained from the furnace is known as black ash, which is cold and washed several times with hot water. To prepare white sodium sulfide crystals, 0.5% sodium cyanide is added to the liquid before crystallization.
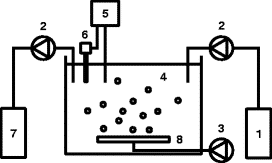
Sodium Sulfide Uses:
- In the manufacture of sulfur textile dyes and in the dyeing of textiles and rayon. (Copper sulfate can also be used as an alternative)
- In the leather industry. (sodium sulfate can also be used as an alternative)

- In the paper industry, for example in the production of writing papers and newspapers or magazines.
- As a weak reducing agent in some chemical reactions.
- In the agricultural industry as a Parasite killer.
- It plays an important role as a sulfonation and sulfomethylation agent in the chemical industry and the production of rubber chemicals, sulfur dyes, and other chemical compounds.
Safety information:
- Causes eye and skin burns;
- Harmful if swallowed. May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract; burns. May cause dizziness, drowsiness, confusion, weakness, irregular breathing, and unconsciousness;
- May cause central nervous system effects;
- Very toxic to aquatic organisms.


![]()
First-aid measures:
- Skin Contact: Immediately flush skin with water.
- Inhalation: move the person to the fresh air.
- Eye Contact: Rinse eyes with water for at least 15-20 minutes.
- Ingestion: Do not induce vomiting. Get medical aid immediately. Call a poison control center.
Packing and storage:
Store in a cool, dry place. Do not store in direct sunlight. Store in a tightly-closed container. Keep away from strong acids.






Reviews
There are no reviews yet.