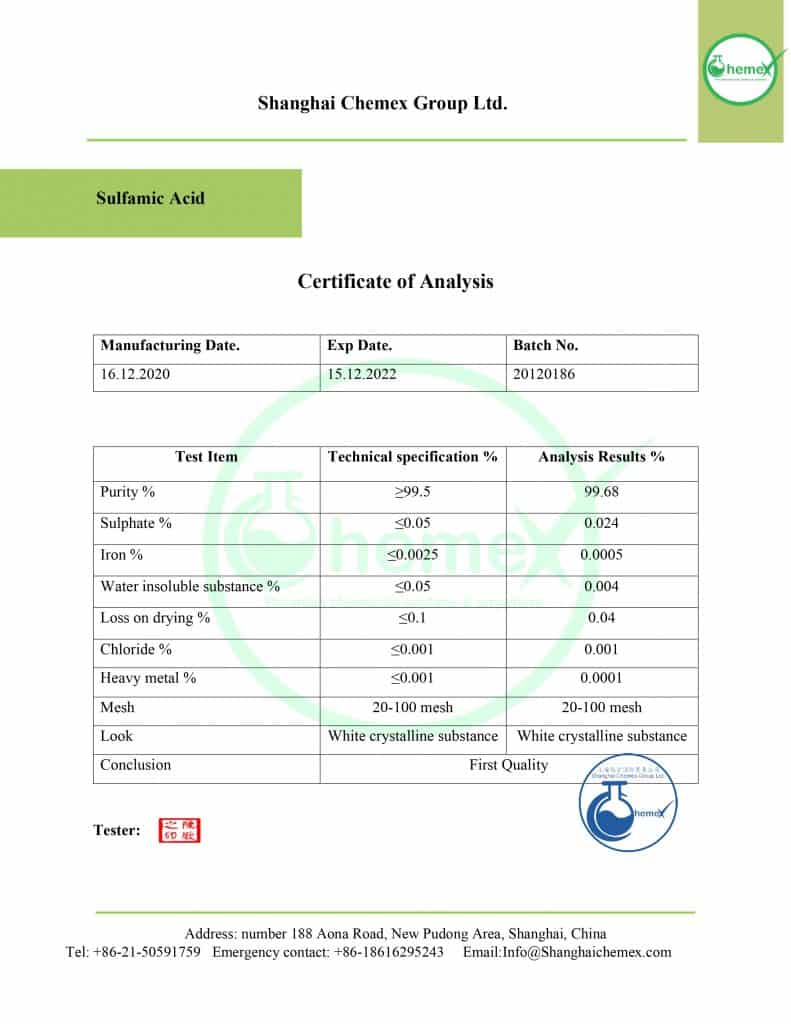
Description
Sulfamic acid is a white, odorless, non-toxic powder with the molecular formula H3NO3S. This acid is one of the relatively strong acids and easily forms sulfamate salts (sulfamic acid derivatives) and is also marketed under the names of sulfamidic acid, amino sulfonic acid, amidosulfonic acid, amidosulfuric acid. This compound is used in the cleaning of metals and ceramics, organic synthesis, paper, and textiles, to control pH, as a water-based cleaner, in plating, pharmaceuticals, and some agricultural products. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
Sulfamic acid appears as white, non-volatile, odorless crystals that are readily soluble in water, alcohols, acetone, and nitrogen-based materials. It can be stored for a long time in laboratory containers, plastic bags, or fibers. The dilute solutions of this material are stable for several months at room temperature.
This material does not ignite easily, but if ignited, it can emit toxic gases. The physical and chemical properties of this material can be summarized in the table below:
| Chemical formula | H3NSO3 |
| Molecular weight (g/mol) | 97.10 |
| Appearance | white crystals |
| Odor | Odorless |
|
Chemical formula
|
H3NSO3 |
| Density (g/cm3) | 2.15 |
| Melting point (°c) | 205 |
| Solubility | Soluble in water, methanol, acetone;
Insoluble in ether |
| Acidity (pKa) | 1 |
Structure of Sulfamic Acid:
Sulfamic acid has a quadrilateral structure and is one of the simplest sulfamic acids. It is composed of a single sulfur atom that is covalently attached to the hydroxide and amino groups by single bonds and to two oxygen atoms by double bonds.
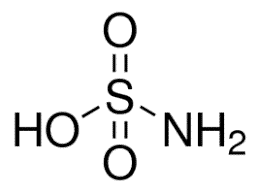
Sulfamic Acid Production Process:
This acid is formed by the reaction of sulfuric acid, sulfur trioxide, and urea. This reaction is highly exothermic and can be dangerous if not done carefully. Various methods have been developed to control the reaction, but have not been very satisfactory due to recovery problems and cooling problems.
The production mechanism of this material is done in two stages. To do this, first, combine urea and sulfuric acid with equal amounts of equivalents and give it time to react. In the next step, the reaction is completed by adding the reaction mixture to a container containing sulfur trioxide.
- OC(NH2)2 + SO3 → OC(NH2)(NHSO3H)
- OC(NH2)(NHSO3H) + H2SO4 → CO2 + 2H3NO3
Sulfamic Acid Uses:
Paper Industry:
Adding this acidic compound stabilizes the chlorine and prevents the paper pulp from decomposing due to the heat of chlorination. This chemical has an effective role in bleaching paper and also reduces the pH without reducing the strength of the paper.
Paint Production:
This compound removes excess nitrides used in diazotization reactions in the production of dyes and pigments.
Chlorination:
This acid can be used in swimming pools or cooling towers due to its chlorine stabilization. like Hydroquinone

Rubber Manufacturing:
It is used in the rubber industry as a baking agent and accelerator of the production process. It has a higher performance than other organic acids and has no limitations on inorganic acids.
Food Industry:
This compound is an additive used in the food industry that is used indirectly and is approved by the FDA.
Cosmetics Industry:
This substance is a cleansing agent that is widely used in the preparation of hair dye.
Buy Sulfamic Acid:
If you are active in industries related to this product, just buy sulfamic acid with a quality guarantee and at the best price. Contact our Shanghai Chemex experts for inventory and selling price of sulfamic acid.
Safety:
sulfamic acid in high concentrations can cause burns, pain, or even corrosion of the skin, so gloves should be used. When this strong acid comes in contact with the skin, immediately wash it thoroughly with soap and water.
If you drink and swallow it, drink two glasses of water or milk and then go to medical centers. Inhalation of this substance can cause cough, shortness of breath, dizziness, and headache. If this happens, go to the open-air immediately and take a deep breath. In more severe cases, you should use oxygen or artificial respiration.
A work environment with this material must be well ventilated, safety must be fully observed and we must use gloves, safety glasses, and special clothing or aprons.
Packaging and Storage:
Store in a cool, dry, and well-ventilated place in closed containers away from incompatible materials.






Reviews
There are no reviews yet.