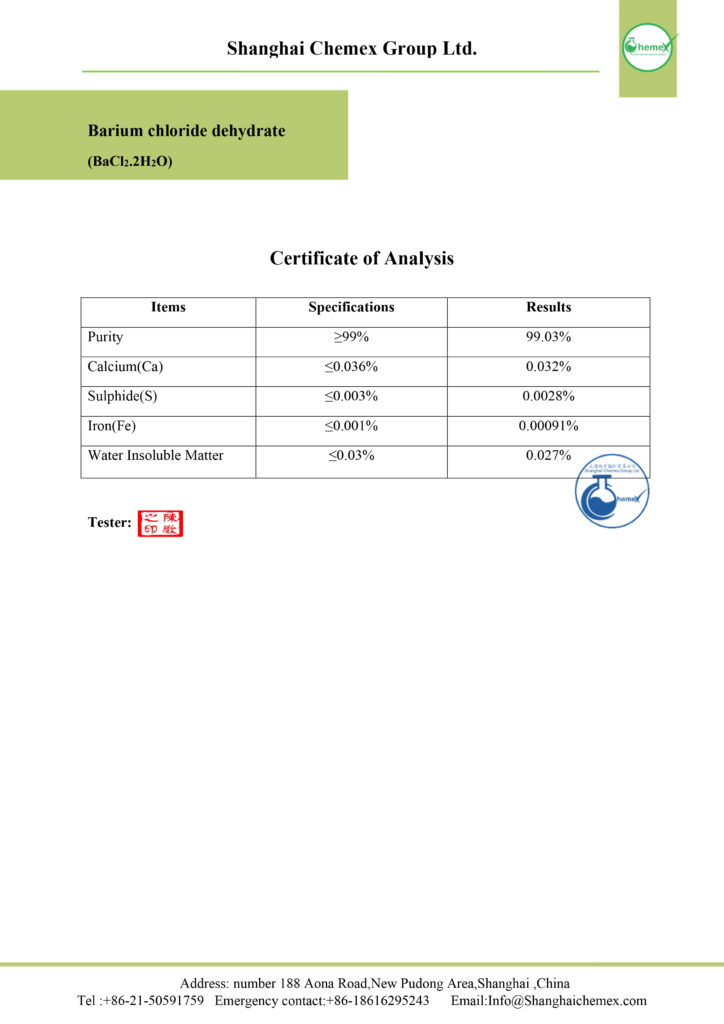
Description
Barium chloride (BaCl2) is one of the most common salts of barium. You might have seen this chemical while looking at the fireworks. When we heat BaCl2, a nice green colour appears, which makes it a perfect choice for a colourful explosion of lights. Barium muriate and barium dichloride are the other names of this salt. As you might have heard, barium salts are considered toxic chemicals. However, several industries use it as a raw material.
How to buy barium chloride?
If you would like to know any further information about barium chloride price or buying this item, our team at Shanghai Chemex will be delighted to help you with that.
Physical and Chemical Properties
Barium chloride with the chemical formula BaCl2 is an ionic compound and one of the most important water-soluble salts. This compound appears as a white, toxic powder that gives the flame a yellow-green colour, but it doesn’t have any detectable odor. Some of the properties are as follows:
| Chemical formula | BaCl2 |
| Barium chloride cas number | 10361-37-2 |
| Molecular Weight (g/mol) | 208.23 (anhydrous)
244.26 (dihydrate) |
| Appearance | White powder |
| Density (g/cm3 at 25 °C) | 3.856 (anhydrous),
3.0979 (dihydrate) |
| Melting point | 962 ° C (1,764 °F; 1,235 K) |
| Boiling point | 1,560 ° C (2,840 °F; 1,830 K) |
| Water Solubility | 37 g/100 cc at 25 °C |
| Solubility | Soluble in methanol, insoluble in ethanol, ethyl acetate |
| Form | Orthorhombic crystals |
| Chemical structure | 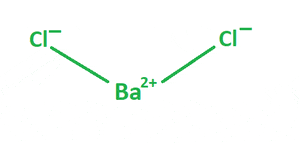 |
Barium Chloride Solubility in water:
BaCl2 can completely dissolve in water and produce aqueous barium chloride, which will dissociate into Ba2+ and Cl–. However, solubility depends on temperature.
| 0 °C | 10 °C | 15 °C | 20 °C | 30 °C | 50 °C | 70 °C | 80 °C | 90 °C |
| 31.2 | 33.5 | 35.8 | 38.1 | 40.8 | 46.2 | 52.5 | 55.8 | 59.4 |
- Is barium Chloride Polar or Nonpolar?
Barium chloride dissolves easily in water and due to its presence in the metal group II, it does not have a 180-degree bond angle. These properties indicate the polarity of this salt.
- Is barium chloride ionic or covalent?
The bond between Ba2+ and Cl– is ionic.
- Barium chloride + aluminium sulfate
When the barium chloride and sulfate ion react together, a white insoluble barium sulfate is produced.
Ba2+(aq) + SO42−(aq) ⇒ BaSO4 (solid)
The Production Process of Barium Chloride:
It is generally produced by the reaction of hydrochloric acid with barium carbonate or barium sulfide. If barium sulfide is used, a very toxic gas, hydrogen sulfide, is produced, which requires additional purification steps. Hydrochloric acid can also cause corrosion problems.
BaSO4 + 4C → BaS + 4CO
BaS + 2HCl → BaCl2 + H2S
Barium Chloride Uses:
Although barium chloride is inexpensive, it has limited applications in industry and laboratories. In fact, the toxicity of this substance reduces its applications.
- It is commonly used in the laboratory to test for sulfate ions.
- Barium chloride produces colored flames when it catches fire, usually yellow and green. This chemical compound is used to produce a variety of incendiary materials for fireworks.

There are different types of barium chloride. One of those types is barium chloride-coated water. This chemical product has stabilizing properties and is used in the manufacture of very strong PVC polymers.
- Production of electronic tools.
- Calibration tools and equipment, quality assurance, quality control.
- It is used in the petroleum industry.
- Used in the steel industry to harden steel.
- Other applications of this material in water treatment and wastewater treatment, oil lubricants, barium chromate, and barium fluoride were mentioned.
Safety Information of Barium Chloride:
Barium chloride is one of the toxic and dangerous chemical compounds for humans. Therefore, its use requires observance of the basic principles of care. Poisoning with this compound causes delirium, esophageal irritation, stomach pain, abdominal cramps, nausea, vomiting, diarrhea, high blood pressure, muscle weakness, vision and speech problems, difficulty breathing, dizziness, tinnitus, and more.
First-aid measures:
- In case of contact with eyes, rinse immediately with plenty of water. If inhaled, remove the poisoned person from the contaminated environment and take them to the open air and contact medical centers. It is also toxic to aquatic life and its antidote is magnesium or sodium sulfate.
- If you swallow barium chloride, rinse your mouth with water and seek medical attention immediately, as the substance dissolves and is rapidly absorbed in water, so symptoms appear quickly and can cause cardiac and respiratory arrest.
- Also, all people in connection with this chemical compound should use special glasses and masks. Because the gases emitted from this chemical compound can be very dangerous and inhalation can be deadly.
Packing and Storage:
You must store this substance in suitable, labeled containers, in a cool, dry, well-ventilated area. keep it away from direct sunlight and incompatible materials.
Frequently asked questions
How toxic is barium chloride?
Using this material without paying attention to safety tips is dangerous because it can decrease the level of potassium in your blood and cause Hypokalemia.
What happens when barium chloride react with water?
BaCl2 can completely dissolve in water. and dissociate to Ba+ and Cl– ions.
What is BaCl2 called?
BaCl2 is a chemical formula of barium chloride. You might have seen this chemical while looking at the fireworks.
What is the pH of barium chloride?
The pH of BaCl2 is about 7. When it dissolves in water, it doesn’t affect the pH of the solution.






Anna –
Is sodium chloride more ionic than barium chloride?
china chemicals –
Yes. sodium chloride is more ionic than BaCl2