Description
Sodium fluoride is a mineral chemical compound with the chemical formula NaF and is used in many industrial, agricultural, medical applications, water treatment, and municipal water fluorides. The positive point of this material is the production of its various forms, so that today, depending on the type of application, it can be prepared in the form of gels, and tablets. Shanghai Chemex is one of the most reputable suppliers of this chemical in the world.

Physical and Chemical Properties:
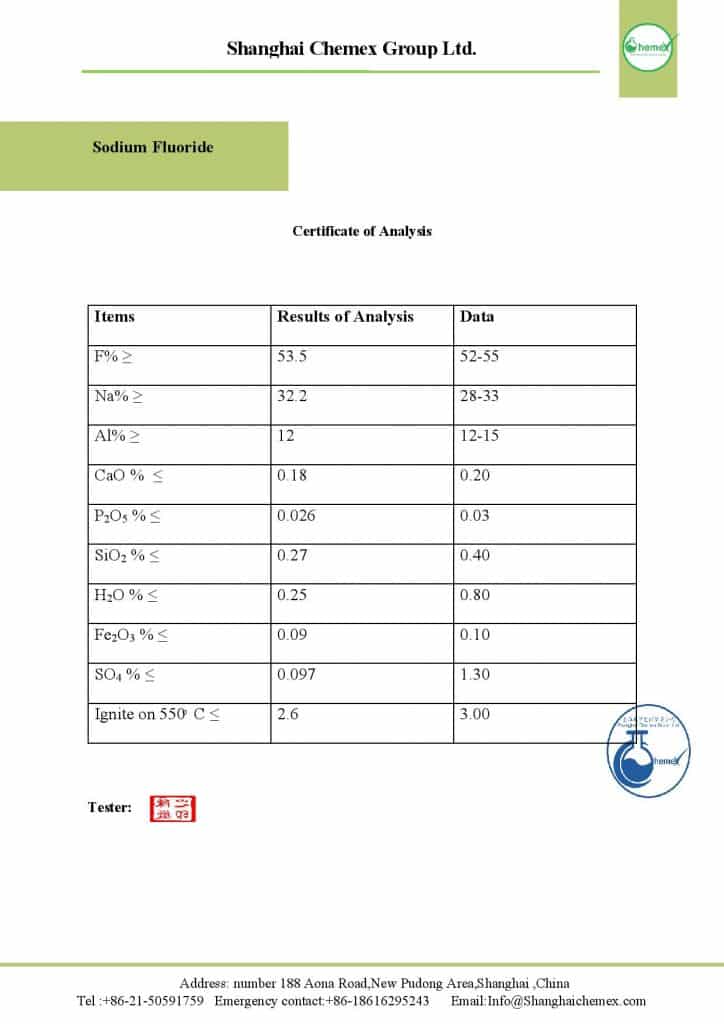
Sodium fluoride is an odorless crystalline solid that appears white and green at room temperature. This product has a high solubility in water and decomposes rapidly into Na ions and fluoride. It is stable under normal conditions, but when heated in the solid state, it decomposes to produce toxic HF gases; The most important physical and chemical properties of this compound can be summarized in the following table:
| IUPAC name | Sodium fluoride |
|
Chemical formula |
NaF |
| Molecular Weight(g/mol) | 41.98 |
| Appearance | Solid |
| PH | 7.4 |
| Odor | odorless |
| Taste | salty |
| Density (g/cm3) | 2.558 |
| Melting point (° C) | 993 |
| Boiling point (° C) | 1,704 |
| Solubility | slightly soluble in water, HF, ammonia
Insoluble in alcohol, acetone, SO2, dimethylformamide |
| Color | White to greenish |
| Form | cubic or tetragonal crystals |
| Chemical Structure Depiction | 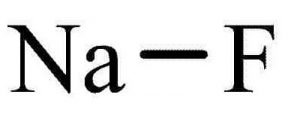 |
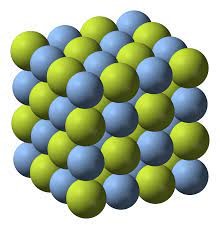
The Structure of Sodium Fluoride:
The crystal structure of sodium fluoride is the same as that of cubic sodium chloride, and both ( Na+) and ( F− ) ions occupy the octahedral space.
Sodium Fluoride Production Process:
This material can be obtained by neutralizing hydrofluoric acid with base materials such as sodium carbonate (Na2CO3) and liquid Sodium hydroxide (NaOH) under controlled pH conditions and proper mixing, in crystals of any size.
HF + NaOH → NaF + H2O
The method of preparation of pure sodium fluoride includes crystallization and washing steps. In the crystallization stage, Na2CO3 is placed in the reactor and hydrofluoric acid is added during stirring. When no carbon dioxide escapes, our process has reached the terminal point. suction filtration is carried out such that crystals are obtained. In the washing step, the crystal is washed twice to obtain pure sodium fluoride.
Sodium Fluoride Uses:
- In general, fluoride can prevent bacterial damage to the teeth and prevent decay. Therefore, this element is used in toothpaste and other oral protection products. Toothpaste containing this product must have permitted levels of NaF. This value is something around 1000 to 1500ppm. Due to the properties of fluoride in tooth protection, it is used as the main composition of mouthwash.

- Because sodium fluoride is toxic, it is commonly used in pesticides, including fungicides, insecticides, and herbicides.
- Due to the effects of this substance in improving auditory nerve dysfunction and reducing the incidence of hearing loss in patients, it has been prescribed tablets containing this compound for these patients.
- The tablets of this product are also used to cure osteoporosis. Sodium fluoride increases bone density but does not reduce the risk of fractures.
Other applications include:
- Used as a fluorescent agent in the synthesis of aromatic compounds.
- In the production of plastic products, Teflon as well as Freon production.
- In engraving on glass.
- Removal of hydrogen fluoride from exhaust gases of various factories.
Global Sodium Fluoride Market:
Geographically, Asia and the Pacific are the largest consumers of sodium fluoride due to their high sales of toothpaste and dental care products. The most important poles of economic industries such as China, India, Japan, and South Korea are the most important consumers of sodium fluoride in the application of wastewater treatment. The two most populous countries, China and India, both located in Asia, are growing rapidly and are seeing rising incomes and purchasing power parity among the general public. North America is another major consumer of sodium fluoride, especially in dental care and wastewater treatment applications. Other countries of the world such as Brazil, Argentina, and South Africa are important applicants for this large market. The European market is expected to grow less than the rest of the world. Of course, with all the uses for sodium fluoride mentioned, increasing awareness of its toxicity in the community has created a constraint on its market.
Buy Sodium Fluoride:
Shanghai Chemex, as a supplier of various chemicals in different grades with the best quality and the most appropriate price, is with you, dear customers. To purchase this product and place an order, please contact our experts.
Safety Information of Sodium Fluoride:
Sodium fluoride is a toxic and corrosive substance and the amount of fluoride used should be 4 mg per day. Side effects include abdominal pain, vomiting, diarrhea, seizures, falls, and even death.
Contact with skin and eyes may result in severe irritation or burns or even death; It is also dangerous for pregnant women and children under 6 years of age because of its destructive effects on the nervous system.


Packing and Storage:
Store in a sealed container in a cool, well-ventilated place at a temperature of 68-77 ° F away from heat and direct sunlight.






Reviews
There are no reviews yet.